|
FAQs on Microscopes, Optical Magnifiers and Aquariums
Related Articles: Infectious
Disease, Infectious Disease 2,
Related FAQs:
Infectious Disease 2,
Biological Cleaners,
Cryptocaryon,
|
Do see the Net, WWM re the QX 2,3,4,...) series of 'scopes by Intel/Mattel |
|
Disease Identification with Photos
5/19/18
Hello Bob and crew!
<Bri! Please re-size and re-send your msg.s WITH MUCH SMALLER files... you've
crashed our mail server. Kbytes, not Mbytes. Thx. Bob Fenner>
Disease Identification 5/19/18
Hello Bob and crew!
<Hey Bri!>
It's been years since I've emailed you! I love using your site as a resource. I
have a purple tang going through tank transfer (1.5 weeks so far) with recurring
white spots. There were no spots for a week, but
yesterday a few appeared again. When I first got the fish, the original spots
were concentrated on the ventral side, with only a couple on the rest of the
body. There were maybe ten total. Fish breathing rate was (and continues to be)
normal. Coloration is good. Appetite is fine. There were no spots for a week,
but overnight a few showed up. There were only five spots on the fish this time,
all concentrated on the left pectoral fin. I decided to clip a section of the
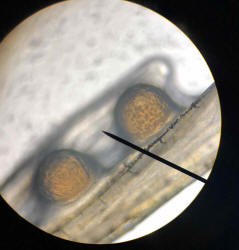
fin and take a look under the microscope.
Attached are photos taken at 10x magnification. I'll try to attach a video as
well.
<Please post the video elsewhere; perhaps YouTube, and send the link to it
instead. We have limited mail server space>
Any thoughts on what this may be?
<From the size... looks too big to be protozoal... Perhaps just accumulation of
body mucus... Happens>
Note in the videos that all movement is created by me changing the focus so you
can see the whole cyst.
The organism was not moving at all and I did not see any cilia or flagella.
<Me neither... is this a dry prep.? That is, was there a slide cover over this
specimen with water around it, supporting it?>
However, I just started treating with Seachem Paraguard 12 hrs before taking the
sample, so maybe these parasites are dead?
<Mmm; maybe, but, could be as stated>
Or eggs of gill/body flukes perhaps?
<Not eggs... would be off the fish's body>
The fish has been treated with PraziPro, but only one round for 2 days. That was
a week ago.
Thank you for sharing your ideas! I'd like to get more specific with my
treatment protocol and your advice is much appreciated!
Lil Bri
<Do try removing the blobs from the spines, scales, put under a cover slip with
a drop of water, and re-shoot and send. Thank you. Bob Fenner>
Re: Disease Identification with Photos 5/19/18
Oops! Sorry! I reformatted/resent the photos, but the video is only 3 seconds
and I can't get it condensed to less than 1.8MB. Hopefully the pictures are
enough for identification purposes! I thought that the
parasite might be Amyloodinium, but it's way too big!
<Yes; too big for any fish parasite I'm aware of>
The photos are only magnified 10x. Then I thought it might be the beginnings of
Lymphocystis from stress?
<Nah; not likely>
It isn't pear shaped like the photos of Cryptocaryon on WetWebMedia, so I'm
guessing not that.
<Agreed. BobF>
|
 |
|
Re: Disease Identification
5/21/18
Thank you for your input! I did prepare the fin clipping as a wet mount, so the
photos are taken with water supporting the body mucus blob.
<Ahh!>
It dried up within about 20mins of taking the photos and the blob shriveled to
about a quarter of its original size. The fish hasn’t shown any more of them, so
I’m unable to take another sample for you so far. I’ll send more photos of the
issue resurfaces. Thanks again!
-Bri
<Thank you. B>
|
|
Re: Goldflake angel white stringy poop
5/18/18
Hi Bob
<Keith>
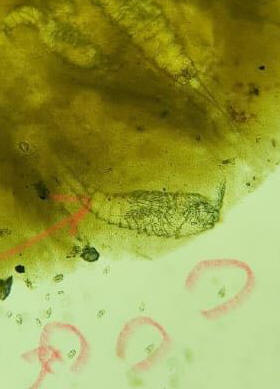
Just an update. I've sent the fish poop for microscopic test and the photos are
attached as below.
<Mmm; can make out the copepods, not the single celled (circled) life>
I was only told that the protozoa are jumping actively. Currently I've re
commenced to dose with Metronidazole and Praziquantel. By looking at the
pictures, am I going the right direction with dosing with Metronidazole or more
should be done? Thank you and much appreciated.
Keith
<Need more resolution... clearer, more close up, resolved pix. Bob Fenner>
|
 full-size crop full-size crop |
Microscope Selection 2/17/17
Good evening,
<Hey Greg>
I have some sort of green phytoplankton in my tank that is making the
water so cloudy I can barely see the back of the tank. I'm pretty sure
it will go away with continued water changes but for the sake of
curiosity, and just in case I have something really nasty on my hands,
I'd like to get a microscope so I can find out exactly what it is. I've
already decided on a USB type so I can send pictures in case I can't
I.D.
<Oh yes... have an Intel Play right here on the shelf in front of me>
it myself. So basically, all I need to know is, how powerful a
scope do I need?
<200-400X>
and any specific models you recommend?
<Yes; please see here:
http://www.wetwebmedia.com/microscopfaqs.htm >
No budget so I'm open to anything.
<What is really needed is incredibly cheap>
While searching your site I came across the mention of there being
digital microscope reviews on Wet Web but I was unable to find them.
<Search... Amazon.com>
So if you could provide a link to these reviews that would be great. But
if not, just providing me the minimum magnification required should
suffice.
<https://www.amazon.com/ThinkGeek-P510002-Digital-Blue-Microscope/dp/B000059TF3>
Thanks a bunch,
Greg
<W. B>
|
Brown Growth Under and Around Zoanthids 12/25/15
Hello crew at WWM,
<Hey Jas>
I hope everyone is having a Merry Christmas!
<And to you and yours>
In the attached
photo, I’m trying to identify the substance that is spreading on the rocks near
the Zoanthid in the middle of the picture. It is brown, translucent and looks
like it has tubes with open holes in spots.
<Yes; my first guess is that this is a sponge of some sort. Second would be an
encrusting brown algae (like Ralfsia); third... oh, I see you mention this
below>
It has spread to some surrounding rocks and has grown on top of some green macro
strands that are attached to the rocks. I am thinking some kind of Tunicate or
Sea Squirt but all the pictures I have seen look more defined than what I have.
I have also considered some type of Sponge or even Cyano but I don’t think a
Sponge
would be so see through and I think that Cyano would be elsewhere in the tank
and would look more slimy.
<The only real way to tell here is to cut a piece off, look under a
microscope... use a reference. Not likely harmful... and will die back given
conditions that favor other life here>
Could it be somehow related to the Zoanthid being they are the same color?
<Mmm; not likely>
On a side note does the polyp on the white frag plug in the bottom left of the
picture look like Corynactis or Pseudocorynactis?
<Possibly the latter>
It’s hard to see but it has a reddish base, clear tentacles, and white balls on
the tips.
Thanks in advance for all your help and for the great service ya’ll provide in
answering questions and giving sound advice with all our aquatic needs. It is
GREATLY APPRECIATED!! Jason
<Cheers! Bob Fenner>
|

 |
|
Re: Brown Growth Under and Around Zoanthids 12/25/15
Bob, Thanks for the quick reply especially since it's Christmas Eve.
<Heeee! Done shopping, and cooking!>
Re: Brown Growth Under and Around Zoanthids 12/25/15
Since you think it's probably harmless, whatever it is, I'll just enjoy what I
have. I don't readily have access to a microscope or I would love to cut a slice
and look underneath one. Jason
<Oh! Do look into the QX series 'scopes.... think they're still about: Amazon?
Yikes; just looked. There's a BUNCH!
Here's the one I have/use:
http://www.amazon.com/ThinkGeek-P510002-Digital-Blue-Microscope/dp/B000059TF3
BobF>
Re: Brown Growth Under and Around Zoanthids 12/25/15
Thanks again Bob, Thanks for the link. I like that it is blue and that you can
hook it up to a PC.
<Yeah; and it has two light sources..... AND you can remove the optical bit and
place it where you want!!!>
Maybe I can talk my wife into getting it for me. Once again have a Merry
Christmas and a Happy New Year!! Jason
<And you and yours Jas. B>
|
Loricariid Disease Question; and microscope use f'
12/9/14
Greetings WetWebMedia,
I've recently adopted a few fish from a friend of a friend who
is draining their pond due to leak in there waterfall and was
possibly leaking into the foundation of their house.(I was bribed with
several bottles of wine to adopt these fish)
<Heeeee! I surrender!>
Included are 2 large albino common Plecs, a male and female and their
offspring( six babies). My concern is brown pigmenting on body
that looks like fungus.
<Mmm; more likely algae (happens) plus some other Protists, Monerans...
not a worry... just need to as smoothly as practical transition these
animals to a warmer, biologically cleaner setting>
Some spots are fairly flush and look like freckling, although some spots
are raised (fleshy appearance) and resemble fungus. I've never seen a
fungus/bacteria of this color. I've also never seen albino Plecs with
brown spots either. What would be the best way to sample/ID.
<A glass slide passed head to tail over the area, scraping off a bit of
this material... not to worry, the scutes of the Loricariid will protect
the fish otherwise. Spreading this in turn on another slide, covering
with a slip, and dabbing a part of a drop of water on the edge of the
slip to prevent drying.... no dying/staining necessary>
I do have a scope powered to X400.
<Great!>
I'm a little new to the whole sampling thing. I've generally used it to
ID parasites. Any chance I could swab the area?
<Yes... there are prep.s, even just the mercury-containing ones used on
humans for topicals... rinsed off after ap.>
My scope maybe underpowered to do any good.
<It is not for most purposes... microbes, culture... distinguishing by
way of are a bit more advanced... that might call for higher power (a K
or two)>
(I am shopping around for a better scope) I should mention the other
fish in pond are a large red Oscar, CAE and swordtail/guppies that look
healthy.
Although filtration had been cut off, I'm sure their were some nitrate
issues IMO. Currently these fish are in a 300g quarantine tank. I am
unable to send a picture. Thank you very much for your time. Aloha
Brandon
<Thank you for such an interesting and informative email. Bob Fenner>
CP use, source of info. on protocol for sampling, microscope
use 6/6/14
Hello! Kathy here again. Thank you for answering my previous question
regarding CP and it breaking down with light. I changed out 25% and
redosed the CP with 1/4 of the original dose. Now I just have one more
question I am hoping you can help me with. I will be redosing with CP
after 10 days of the initial dose. My question is can I dose Cupramine
and CP concurrently?
<Yes; but for what use? Mixing med.s does "weaken the
patients" in most cases>
Cupramine is so easy to test for and I guess I am just afraid that maybe
I am not dosing enough CP and I don't want to dose too much and threes
just no way to test for it. So I was hoping I can dose and maintain my
QT at about .30 of Cupramine while dosing with CP at 40mg per gallon
every 10 days and also adding PraziPro once every 7 days. Is this ok to
do?
<Can be done>
Also I will be purchasing a microscope
<Ah, good!>
and was wondering where I can find the best information regarding how to
skin scrape fish to check under the scope and pictures on all the
diseases the way they look under the microscope. Thanks again in advance
for your help.
<The single best reference is Ed Noga's "Fish Disease; Diagnosis and
Treatment"... first or second edition. Can be purchased as an eBook on
Amazon I believe; perhaps borrowed through a library service. BobF>
Re: Marine Velvet / Ich and
Chloroquine/Hypo Treatment 6/6/14
Hi Bob,
<Brad>
I've attached a video I recorded from my microscope of some skin/gill
scrapings. To be honest I'm not sure if anything looks like what I see
in reference books. The video is shot with a 40x lenses and the USB
eyepiece is 10x. At 100 or 200x I can't see anything moving, but at 400x
I can see all sorts of activity. I'm not sure if this is parasites or
just bacteria and microfauna instead?
<Too big to be bacteria...>
There is one larger object which appears to be alive and moving, but not
sure if that might be a fluke?
<Might be... I think I see a haptor...
Video linked here:>
I don't see anything that looks like Velvet or Ich.
<Me neither>
If you have any guidance on what this might be (if anything (I'd
appreciate it) or if you want me to take a different type of sample let
me know. I do have a staining kit and oil immersion, just haven't done
much of this before.
Best Regards,
Brad
<Google: Trematode haptor... B>
|
microscopic images
1018/13
Hi Bob,
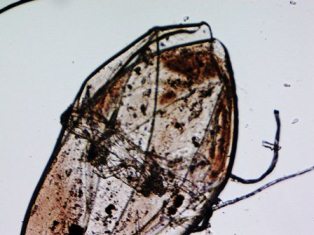
I recently did a freshwater dip on a fish and found something. I
looked at it through the microscope. Would it be possible for you
to identify it?
Thank you.
Jennifer
<... this just fell off the fish presumably? What magnification is this?
Be all as it may/is, I can't make out what this might be. Cheers, Bob
Fenner>.
|
.jpg) |
|
Re: microscopic images
1018/13
Hi Bob,
It is 4x. I couldn't fit it all on one image. It did fall off the fish. I
was wondering if it could be hexamita. Thank you for looking.
<Mmm, no; not this large... likely this is a couple of overlapping
scales.
BobF>
Jennifer
Re: microscopic images
1018/13
Thank you for looking. I've been looking into an alternate to flukes
which
I thought had been plaguing my flame angel. He lays on his side under
the
filter. The color in his face is faded and sometimes stringy feces. I
thought hexamita might have been the culprit.
<... likely "just" stress, manifestations thereof. Many fishes exposed
to
treatments, moved about et al. show such... e.g. how hematocrits... And
very important for them to have high packed-cell volume, RBCs... as the
availability of oxygen in solution is small...>
I took the pink spotted goby to the head aquarist at our local marine
science center because he has been flashing (the fish, not the
aquarist).
He did a scrape and a fin clip, no flukes found.
<Mmm, I'd go slow on moving my fish about>
Thanks again, Bob! Have a good night:)
Jennifer
<Ah, you as well. Am headed out in the early AM to CT to give some talks
at a friend's store, install co. BobF>
Re: microscopic images 10/20/13
Hi Bob,
At this point fish are just being watched, fed a good diet and water
changes. The goby is still flashing. I'm wondering if it is just him
healing from where the flukes haptors were dug in to his skin. At least
that is what I'm hoping.
Hope all goes/went well in CT:)
Jennifer
<Ah yes; mighty fine. B>
|
|
Little 'bugs' swarming on star polyp 12/5/11
I am sooo grateful to you folks, and I hope I'm not wearing
out my welcome. But I am constantly running into
curiosities.
<Life is grand eh?>
My star polyp which I've had for about 5 weeks is swarming
with little 'bugs' of some sort. They don't
seem to be doing any damage, but they still make me nervous.
Before I mashed a captive onto a slide, I was able to (barely due
to its tiny size) note a few things.
It is about 3-4 mm long and perhaps 1/2 mm in diameter.
It is very light tan, somewhat transparent
It has two very long antennae, two shorter antenna-like
things, six legs (not positive of this), and a funny pronged
tail.
They scurry in fast, short bursts and dive into holes in
the rock if frightened.
<Mmm, yep>
I apologize for the poor photo, but my microscope does not have
low enough magnification to get it in one shot. So I had to
take three shots and crudely stitch them together.
<Fab...!>
Any idea what this is? I assumed it was some sort of
amphipod, but I looked at a huge number of amphipod photos on the
web and none of them looked anything like this to me.
<Looks to be what you state, Scud! An Amphipod member>
Thanks!
Tim
<Neat animals to have. Bob Fenner>
|
|
/Amphipods/img23.gif)
Re: Little 'bugs' swarming on star polyp --
12/5/11
Bob - Thank you!
<Welcome Tim>
I am having a great time learning new things with this
seemingly endless hobby.
<Beyond my life time for sure>
By the way, I have to thank you for that microscope photo of the
amphipod that I sent. You probably don't remember, with
all the emails you answer.
But early this year, shortly after I stocked my first tank and
asked you a question based on a crude verbal description of
something emerging from live rock, you encouraged me to get a
digital microscope. I did so then, and it has opened up a
whole new world. Thank you! I live just 15 miles from
a state university, and I inquired there to see if I could audit
a marine biology course, but they don't have a single marine
biologist on the faculty! Arrgh.
Tim
<Let's change this situation to Ahh! Cheers, BobF>
|
Re: Update: Is the mystery guest really Sporadotrema?
Microscopes 2/6/11
Interesting... Alrighty, I do not have such a microscope but I am lucky
in that I can certainly obtain one and I have a husband who
wouldn't blink twice at such an acquisition.
<Yay!>
Any suggestions on what would be appropriate?
<Mmm, yes... do see the Net, WWM re the QX (2,3,4,...) series of
'scopes by Intel/Mattel... is what I use, have on my desk here...
in addn. to some other old light microscopes>
And once I have such a thing, what is it that I would need to do?
<Mmm, take a look at a sample... if one of the above scopes,
you'll be able to take pix, even kinetic... share re ID>
I assume obtain a piece of the creature in question and take a peek
under the microscope? These little guys are pretty "stuck" to
the rock - do I also need some sort of sharp object with which to
remove enough to examine?
<Yes... an X-acto knife or other sharp implement>
This is fun! :-)
<I'll say!>
Thanks!!!
Lori
<Thank you Lori. BobF>
Assisted Viewing (Magnifiers) -- 09/04/08 Are there
some kinds of magnifying devices that will enable me to see all the
interesting things in my tank in greater detail?
<<Indeed'¦ At the low end of the spectrum there's a
gadget called the reef porthole (see here:
http://www.petdiscounters.com/The-Reef-Porthole-Aquarium-Magnifying-Lens-p7021.html).
But if you REALLY want to see things of interest, then invest in a
Mesoscope (see here:
http://captainaquarium.com/index.php?main_page=product_info&products_id=119)
>> Thank you, Margo <<Happy to assist. EricR>>
The microscopic world, speculations on FW, SW "Dead
Sea" effect 11/15/05 Hello again, <Hi there>
Jonathan here. Have acquired an Observer III microscope to help me
diagnose fish problems where I work. Both of the fresh and the salt.
<Another world awaits you> I can do 40x, 100x, and 400x. I'll
be getting another eyepiece so i can do 600x for that just a little
bigger than 400x. <Mmm, much larger> I've been making dark
field / oblique filters to try and see what i see. <... I, not
"i"> I don't want to invest in phase contrast just
yet, unless I find out there's no other way. Have you ever gotten
decent resolution for searching for parasites at 400x with a dark field
filter? <Yes> I have to use oblique by slightly moving my filter
holder out alignment. That and sometimes giving myself a headache by
closing down the iris aperture all the way. I'll be getting a
mount for a digital camera, so that I may attach it to the scope. May I
send you an video for feedback? <Yes> I may make a website to
share my progress with others. <Outstanding> I'll keep
learning where I am, and try and take a course at my college to refresh
my technique. I might have an opportunity to attend the diseases of
warm water fish seminar in Florida. Do you think it would be an
improving experience? <Yes> Or that by working in an aquarium
store that I'll eventually see most of what they would show me.
<Oh no... a very good idea to have both experiences to draw from>
Two recurrent problems, which may even be related, in salt; is possible
Brooklynella running loose and cloudy eye. Coppersafe is at half dose
continuously. Brook is said not to be affected by copper sulfate, which
would make sense. The way it looks on the fish is very much the
description in books and internet. Have scoped a few scrapes, but
I'm too new to say "that's it". I'm taking
action against it, but victory is not yet reached. [course of
action is freshwater baths sometimes with Meth blue 7 -15 min.s every 3
days about, but return to the same tank. I can't pull a clean tank
out of the air, <...? But you can buy a scope?> and by corporate
all tanks need to be full, ha. I could shut off a tank from the
central, remove the copper, and hit it with Rid Ich+, which I'm
considering, if my bath approach is not getting results..] <Shotgun
approaches are not encouraged> Would it be possible that ich or
velvet could be present in the gills of new fish at such a level to
cause death without being present at all on the body & treating at
half dose of copper is not enough to solve the problem? <Yes... a
therapeutic dose is just that... less than is more harm than good>
Its a possibility I just recently considered. I think if I see encysted
ich or velvet on a newly introduced fish, its probably just temporary.
Until it falls off divides and the copper kills the free swimming
stage. Cloudy eye I think is caused by our water. Most things I read
linked it to environmental issues. Our nitrate is barley registering on
our Jungle Quick dip stick, as accurate as that is. I think we may be
exporting nitrate by scrubbing algae, and removing and drying out Cyano
infested crush coral substrate. <Your speculation is
worthy> So that nitrate would not be an entirely accurate judge of
the water quality. Only doing 30 gallons out every week or two, may not
adequate in a 900 gallon system. <Uh, no> I think we actually
evaporating and topping off more than we are taking out and replacing.
I've noted this on a discus tank we had by using a TDS meter. The
TDS value was much higher in the tank than the source water like 3 - 4
times. <Like potted houseplants, these tanks need periodic large
water change-outs to dilute solids...> Even taking account whatever
live plants died or bogwood adds, it gave me a way of showing the
problem. Ha, the TDS wouldn't work in saltwater, its over its
limit. If hypothetically this we evaporating and replacing say 75
gallons a week, and only did a 30 gallon water change a week. Could
this lead to problems? <Yes> Wouldn't over time
the whole of water become more mineral rich, and with all the
contaminants of the tap. This might lead to a cloudy eye problem.
<Agreed> Too much contaminants, too much minerals, too much
bacteria supported by those. Any ideas? I've tried to keep
this short. Sorry and thanks, Jonathan <Do please learn to/use your
spelling and grammar checking tools... a good learning experience. Bob
Fenner>
|
|

