FAQs about Stony Coral Health/Disease/Pests: Social
(Allelopathy mostly)Related Articles: Coral Pests and Disease; pests, predators,
diseases and conditions by Sara Mavinkurve,
Quarantine of Corals
and Invertebrates, LPS
Corals, True or Stony Corals, Order
Scleractinia, Propagation for Marine
Aquarium Use,
Related FAQs: Stony Coral Disease 1, Stony Coral Disease 2, Stony Coral Disease 3, Stony Coral Disease 4, Stony Coral Disease 5, Stony Coral Disease 6, Stony Coral Disease 7, Stony Coral Disease 8, Stony Coral Disease 9, Stony Coral Disease 10,
Stony Coral Disease 11, Stony Coral Disease
12, Stony Coral Disease 13,
Stony Coral Disease 14,
FAQs on Stony Coral Disease by Category: Diagnosing:
Environmental (Pollution/Poisoning, Lighting...),
Nutritional, Trauma,
Pathogenic (Infectious, Parasitic, Viral)
Predatory/Pest,
Treatments
FAQs on Stony Coral Disease by Family: Acroporid Disease, Acroporid Disease 2, Acroporid Disease 3, Acroporid Disease 4..., Caryophyllid Disease, Caryophyllid Disease 2..., Elegance Coral Disease/Pests, Dendrophylliid Disease, Faviid Disease, Faviid Disease 2, Fungiid Disease, Mussid Disease, Mussid Health 2, Poritid Health, Trachyphylliid Disease, Trachyphyllia Disease 2,
FAQs on Stony Coral Disease by Type: Brown Jelly Disease, RTN,
|
Order of introduction, slowly (weeks) acclimating new
specimens to an established/occupied system via water interchanges...
Almost eliminates entirely incidents of overt allelopathy
Well; there are a few paths, steps you can take to alleviate the
allelopathy... Read here re:
http://www.wetwebmedia.com/cnidcompppt.htm
|
Please help. Cnid. allelopathy in a new, large sys.
5/26/16
Hello Mr. Fenner,
<Hey Dai>
I hope you can point me in the right direction. I have a 265 gallon reef
tank that I set up 4 months ago. I have sump/refugium, 4" carbon
reactor, 6" media reactor with media pellets, 8" skimmer. Lights are 4 Hydra 26
HDs.
Nitrate /phosphate is zero
<Mmm; stop here: You know that all bio-mineralizing life requires "some" NO3 and
HPO4? W/o these basic chemical nutrients all your "corals" will be very
stressed/starved>
and the water is within reef parameters. The tank has 3 sections. Left
is Zoas, center is 100 plus heads of hammers/frogspawn, and the right is 18"
green leather and 4" green toadstool.
<Yeeikes....>
Everything was fine with Zoas multiplying, hammers sprouts tiny babies but
within the past 5 days, some of the hammer heads just died leaving stalk
white skeletons.
<The "losers"... to either the Alcyoniids or Zoanthids>
I bought a 60 gallon so this weekend I can put the finger/toadstool in it.
I hook up the FX6 filter (400 GPH) with carbon to address chemical warfare since
Monday. I think the reasons on the demise of the hammers are :
1. Chemical release from leather/toadstool.
<Possibly>
2. Media reactor strips all nitrate/phosphate which hammers do need to grow.
<Definitely a/some factor>
So my plan is:
1. Move the toadstool/green finger to the 60 gallon.
<Okay>
2. Discontinue the FX6.
<All-right>
3. Discontinue the carbon and media reactor (all in one pellets).
<Sounds good>
The Zoas are thriving with new heads forming every week. I love to have a garden
of hammers and while some people grow these like weeds, I can't keep them alive.
Before dying, they thrive then die next day. Is my diagnosis and plan of action
correct? Thank you Mr. Fenner. Dai
<I do agree with your plan; is what I would do, try at this point. IF no
improvement, I would move the Euphyllias elsewhere. DO PLEASE READ AND HEED my
acclimation protocol for introducing any/all NEW Cnidarians... by mixing water
to/fro twixt the main-display and isolation/quarantine system. HERE:
http://www.wetwebmedia.com/CorlCompArt.htm
Bob Fenner>
<<Note: next time mention triple-dosing iodide-ate>>
Re: Please help
Thank you Mr. Fenner. So you are saying maybe it is the Zoas that are affecting
the hammers as well ?
<Might/could well be; yes>
In that case should I move the hammers to the 60 and leave the toadstool/finger
in the main tank?
<Yes; a better plan... Plus I'd triple dose all (both systems) with
iodide-ate... every three days, three times>
Or just leave them in the main tank for now? I bought the 60 for the leathers
specifically. Is it OK to run the 4" carbon reactor?
<Can't say from here. I would NOT use such on a newish system period>
On another topic. I think the" all in one pellets" give people the false sense
of security.
<Oh yeah; the/a "western ethic"... trained to be good consumers... "Buying"
something... but sans understanding, often false notion/s>
The dealer touts as " zero nitrate and zero phosphate" so people go crazy
thinking they don't have to do water for a year. But while this is true, it is
hurting corals because it is stripping of the essential nutrients that corals
need.
<Yes... even other media/sources tout that the world's reefs are "nutrient free"
when in good shape. NOT the case. They are nutrient concentrated; with the life
there scavenging most all available. NEVER zero nutrients in the water>
If you have to rely on these 'miracles" to get nitrate/PO4 to be zero then that
person needs to evaluate his technique of husbandry.
<Very well stated>
Thank you and I look forward to your guidance. Dai
<And I to your further sharing. BobF>
Re: Please help 5/27/16
Ok, so this is the plan. Move the hammers to the 60. How much of new water to
old water ratio be?
<About half>
If the old water is not good (chemical warfare, no nutrients) then may be start
with 100% fresh water?
<Not I>
You talk about triple dose 2 tanks but I am not familiar with the medication.
You mean every three days, I dose the tank 3 times a day and for how long? Is
this the iodine coral dip?
<See WWM re. B>
Thanks! Dai
Sponge Trouble (with Stony Corals), moved from FB
12/10/14
Morning Bob! I was wondering if I could pick your brain about chemical warfare
and corals.
I'm presently contending with a scenario where water parameters are spot on and
stable yet Euphyllia that once thrived are
now receding at a rapid rate. Upon removal the bases of 90% of the colonies were
found to be encrusted with numerous sponges.
Sponge tissue was scrubbed off and colonies were given an iodine dip. Now how to
contend with the remaining sponges that
have set up shop amongst the rock? Possible that the sponges are part of the
problem here? Am I losing my mind? Thanks Bob!
Jon Tarutis
House of Fins, a few seconds ago
Oh! Jon; pls send all petfish related mat. to me via Crew@WetWebMedia.comJust
cut/paste this. Ah yes; but/and do def. know of the
troubles w/ Euphylliids (and other stonies) and sponges (underneath). NEED to
removed the colonies and moved elsewhere.
Am cc'ing a friend (Cam Bee out working w/ Walt Smith in FJ and ChrisT who used
to, who both have extraordinary olfaction,
can/do smell the sponges... and reject specimens collected w/ them. Hopeful they
will share input w/ you here re.
Bob
|
Multiple coral health issues - allelopathy?
6/6/13
Hello
<Hi there>
I have multiple corals in some type of distress at the moment and I
have not seen an obvious chemical or physical cause. I have
gone the first 10 months with zero issues, now this has popped up.
I have searched Borneman's book, your site, and the internet and
have not figured it out for myself. Perhaps you can help.
<Let's hope so>
Tank: 46 gallon, mixed reef, about one year old.
I have about one year's experience as a marine aquarist. Remora
skimmer, carbon and media in a canister filter. Carbon change
every 2 months (most recent one month ago), water change 10% every 2
weeks (Reef Crystals). Reef Fusion 2 part a couple times a week is
the only additive.
<Do you measure [Ca] and alkalinity? Mg concentration in balance?>
Water source is a deep private well with no chlorine or nitrates.
Fish: Green Chromis, midas blenny, royal gramma, Firefish, flame
angelfish.
Inverts: porcelain crab,
<Is this animal crawling over your corals?>
pom pom crab, cleaner shrimp, sexy shrimp (2), long spined urchin, dwarf
hermits (4), various snails, feather dusters (2)
Corals: Zoas/palys, toadstools, xenia, mushrooms, yellow
leather, bubble, chalice, monti's, small hammer, candy canes, finger
leather. All corals except those mentioned below appear healthy.
<Mmmm>
Parameters:
temp: 76
s.g.: 1.024-1.026
Ca: 420ppm
<Need to know [Mg]>
kH: 9
Nitrate: 0
<... need>
Phosphate: 0 (or very close)
<... your photosynthates need appreciable/measurable NO3 and HPO4...
can't live w/o>
Ammonia: 0
Issue:
1) Two candy cane colonies look very unhealthy. The heads
began to look clear and saggy, with the tentacles in affected areas
absent. Within the last week a neon slime has appeared in parts of the
polyp. The polyps are normally mint green, no neon at all.
At the same time, an adjacent neon green candy cane seems unaffected
even though a few heads were touching the sick coral. I have moved
it away.
<I see this>
2) A largish finger leather which is nearby to the affected candy cane
no longer expands much during the day. This has been about 2
weeks. In the past we would see this for a couple days then it
would shed some mucous and come back to it's original size. This
seems different.3) A Monti undata, also nearby, has changed in
appearance, becoming wrinkly and bumpy instead of smooth. It used
to be covered in visible white polyps, these have all but disappeared.
4) A Monti cap, also nearby, also had it's polyps disappear. It
used to look fuzzy, now it looks very hard. It has kept its color,
as has the undata.
5) A finger leather frag died. This was fragged off of the main
finger leather in the tank.
Possible causes:
1) Lighting. I changed lighting in February, from 96w T5
to 120w controllable led. I acclimated very slowly, stating at 40%
strength, increasing by 5% every few days.
<Mmm; not likely a principal source of trouble>
2) Allelopathy. In February I fragged the finger leather
as it was getting too close to the candy canes. Fragged
outside of DT, and kept frags and parent in QT until they were healed
about a week. Although all these issues are on the side of the tank
where the finger leather lives, I don't know why this would come on
suddenly. The candy canes were added last August, the finger
leather last September. No change in their locations since. After
reading through your advice I think you will say that this is the cause.
<Could well be>
3) Parasites/disease. I do not see anything on them. All
heads appear to be affected equally.
<I discount this as well>
The finger leather may be the culprit, but I hate to pull it out of the
tank without knowing for sure. Perhaps you have seen a candy cane
coral with this appearance before?
<Yes; due to allelopathy... chemical and physical warfare w/ other
Cnidarians>
Are they capable of recovering at this point?
<Oh yes; certainly>
Any help would be appreciated. Thank you for your time.
Paul
<Well; there are a few paths, steps you can take to alleviate the
allelopathy... Read here re:
http://www.wetwebmedia.com/cnidcompppt.htm
and the linked files above... Again, you want to allow (likely by simply
stepping up feeding) some NO3 and HPO4. Bob Fenner>
|
.jpg)
.jpg) |
|
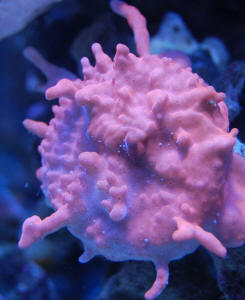
Tissue Necrosis, Discoloration, and White Excretions
on SPS/LPS 4/21/10
Hi there and thanks in advance.
<Welcome Aaron>
I've recently run into some trouble with my year old 47g
mixed reef. Over the past few days I've noticed some
startling tissue necrosis and zero polyp extension as well as
general paling out of colors on a relatively localized
section of my display.
<I see this>
The affected corals include Cyphastrea, a Favites (possibly),
several encrusting Montiporas, two Acropora colonies. In addition
to the tissue necrosis I've noticed a white,
mucus/string-like excretion from the stony corals. I was careful
to verify this wasn't a Nudibranch or anything living.
Several weeks prior I added some clove polyps without dipping
them (I'm ashamed to say) but would think that if this was
something I had introduced with them it wouldn't be affecting
such a wide range of coral in similar ways.
<Likely just an interaction (allelopathy) w/ the Clavulariids
period...
Read here: http://wetwebmedia.com/polypcompfaqs.htm
Perhaps a "cascade event" twixt them, the Zoanthids,
Euphyllia in turn to all>
I should note that a frogspawn, numerous Zoanthids and several
other LPS corals, as well as a Pocillopora are in the general
area and not exhibiting symptoms.
<The "winners">
I also ran a battery of tests with the following results:
Salinity: 1.025 Ammonia: 0ppm, Nitrite: 0ppm, Nitrate: 5ppm, Mg:
1200ppm, Ca: 440ppm, Alk: 5.9 dKH, pH: 7.8. Further investigation
yielded a heater plugged into a surge protector which had been
turned off/blown within the last few days (not quite sure when).
Thankfully, the ambient temperature is around 75 but it is likely
that there were some temperature swings over night. Despite the
low pH and alkalinity levels (which are being rectified), I'm
not certain that the parameters or temperature is causing the
problems as there are numerous corals (various Acroporas,
Blastomussa, xenia, clams) that I would have thought to be more
fragile in other portions of the tank that as of yet appear to be
doing just fine.
I'm hoping for some sort of recommendation for treatment.
<... chemical filtration, water changes... taking out the most
likely offenders, slowly re-acclimating them through
water-mixing... Read here: http://wetwebmedia.com/cnidcompppt.htm
and the linked files above>
Obviously getting the parameters back in check is a priority (and
I'm hoping your opinion is that this is the root cause as
it's easily fixed!). I did a 30% water change yesterday and
plan to continue this for a number of days in case something was
accidentally added to the water (although, again, the localized
nature of the problem makes me wonder whether that could be the
case.
Unfortunately, a freshwater dip for the corals would be quite
difficult to manage as many of them are encrusted on a very large
rock but if it meant containing the spread of something
potentially lethal to my entire aquarium, I could find a way.
I have attached pictures of several of the affected corals for
your inspection. Please advise!
Thank you,
Aaron
<The most likely scenario/cause here is Cnidarian
allelopathy... Read where you are referred to. Bob Fenner>
|
 |
Stony issues, hlth... allelopathic cascade event
likely 10/21/08 I have two main issues: 1)
injuries on my tongue coral and pagoda cup; and, 2) tissue recession
and/or bleaching on my Pocillopora and my Hydnophora. I have a 150 gal
aquarium with a 30 gal refugium, 3x150W HQI (10,000K) + 4x96W (420nm)
Actinic, bought new 14 months ago. Calcium is 400, Alkalinity 3.0, Temp
78.5 (controlled by chiller), pH 8.2 (relatively constant, as we have
an automatic reef doser, with two-part A and B solution). <I take it
the last are administered to the refugium or in/with new water during
change-outs> We have had our tank for fourteen months, and have had
no problems. Most of the corals in the tank have come from our previous
smaller tanks over the last five years. For instance, the tongue coral
and the pagoda cup are over four years old, and we moved them into our
consolidated 150-gal August 2007. Here's the problem with those two
- they were stung by a falling frogspawn. <Ooohhh, can be fatal>
It fell and touched the edge of each coral, leaving a visible injury.
The tissue has decayed, and I have noticed my cleaner shrimp and
blue-tipped hermit crabs picking at the injuries. I moved the tongue
into my quarantine tank under PC lighting, and I'm considering
moving the pagoda cup. Is this course of action recommended? <Mmm,
to avoid the picking mentioned? Actually not if the removal by these
crustaceans is/was only the damaged tissue> My idea is, without the
shrimp and crabs picking on them, the tissue will grow back, and they
can be returned to my display tank. <Mmm... I'd rather had you
cover the corals in place... with inverted "strawberry
baskets" or such> Please note that this is not brown jelly, and
the dead tissue area affected reveals the underlying white skeleton.
The area in question on each one is about one square inch, relative to
approximately 10 square inches of total healthy coral. The polyps are
extending, and the rest of the coral looks great. Please let me know
your opinion on this LPS injury problem. <Very common... all else
being maintained, usually not fatal> Regarding my second issue,
above, over the last week, my Pocillopora, which is over a year old,
and has been thriving (growing, polyps extended, branching), has
started to bleach from the inside out (ie the middle of the coral has
turned white, while the tips have retained their color and polyp
expansion). However, the problem seems to be getting worse. I am
thinking about fragging this coral, as it is medium size. Should I do
this, and what is the likely cause of this tissue
necrosis/bleaching/recession? <Nothing obvious here... perhaps (the
most likely guess) a result of the reaction of the other two
Scleractinians to being stung by the Euphyllia> Because the
temperature and other water parameters are stable, and the coral has
not been moved since we've had it, could it be due to decreased
light intensity from aging bulbs? <Doubtful if the other stony
corals are unaffected> Also, we recently switched our A&B
solution to C-Balance from Aquaphilic. Could this also be a
contributing factor? <Again, doubtful... this variously diluted
Peter Wilkens/Two Li'l Fishies product has been implicated (due to
sloppy, inconsistent dilution) at times... but not recently> We
switched it on the first of the month, and have just developed problems
over the last week. I do not want to lose this coral, as we have had it
for over a year, and it has been thriving, along with the rest of our
tank, which has over forty species of soft and stony corals. All other
corals have great polyp expansion, and are growing and healthy, at this
time. However, this morning I awoke to find a small area of bleaching
on my otherwise healthy (and huge) Hydnophora, which we have had nearly
five years, and which has over doubled in size (larger than a bowling
ball) since we put it in the new tank about 14 months ago. One of the
fingers only has bleached at the tip. Should I cut that finger off
before it spreads? <Mmm> Or should I leave it alone and hope it
grows back. <I would do this last> Could the Pocillopora and
Hydnophora have the same problem? <Yes> What is the recommended
course of action for both of these corals? <Patience... and reading:
http://wetwebmedia.com/cnidcompppt.htm and the linked files above>
p.s. We haven't lost any corals, or had any problems whatsoever,
since we set up our 150-gal over 14 months ago, from five smaller tanks
that we had kept over the previous five years. Please help, so we
don't lose the ones that are hurting. P.P.S. It may help you to
know that we have six fish (3 tangs, a blenny, a clown, and a royal
gramma), and some inverts (snails, blue-tipped hermit crabs, a cleaner
shrimp, and a coral banded shrimp), which all live peacefully together.
No other corals are in the vicinity of the problem corals, and no other
corals appear to have any issues at this time. We are preparing to do a
15% water change, and await your response. Thank you in advance for
your help. <Please do keep me/us abreast of further
developments/observations. Bob Fenner>
| How to identify what is killing these 2 Corals...
Actinarian allelopathy, lack of reading
3/3/08 Hi Crew, <Sammy> I have 2 corals, a Horn Coral and
a Moon Coral, in my 175 gal tank that seems to be slowing dying and
I am hoping you can point me to what I should be looking for.
<Uhh, there's something else here... in the upper left... an
Actinarian...> Here is a view of the tank showing the position
of both corals. Here are closer up shots of the 2 corals. Here, you
can see clearly that sections are completely dead. Part of it has
the purple coralline algae growing on it already. Below is a shot
taken in mid November and it was in much better shape, although
there were already signs of decay in the shadowed parts. This moon
coral was placed on the ledge under the anemone to the left of the
1st photo. <Uhh, yes...> As the anemone grew, it was getting
too close to the moon coral causing one edge to die. <Yes, and
that's not all> I have moved it 2 months ago to this
location. But the decay seems to be progressing, especially towards
the top left side. Here is an older shot taken end of last November
when it was still placed under the anemone. <A mistake> Both
corals are under 14K halide almost directly and getting lots of
light. Water parameters seems to be normal, with calcium at 440ppm,
NO3 ~10, pH 8.3, temp 76F. I had a calcium reactor running about 3
months ago and I stopped dosing iodine and strontium. Should I
continue to do so? <What do your tests for these show?> I am
using Carib Sea Aragonite and I thought it has iodine in it.
<... no> Another change was the addition of a Sea Apple 4
weeks ago, as you can see towards the lower right of the first
photo. Since adding the sea apple, I have been feeding 30cc Phyto
Feast once a day. I wonder if the Sea Apple <Toxic...> is
poisoning these 2 corals. <Not likely... all would be dead>
My other corals seem to be fine, however. One other thing I noticed
is that all my string worms have gone hiding from the surface of
the sand since the addition of the Sea Apple. I don't know if
it is the Sea Apple or the frequent feeding of Phyto Feast. I have
reasonably good water flow and the Sea Apple is quite far away from
these two corals. Another thing I have noticed is this coral. This
shot was taken at night, so the polyps have already retracted. But
you can see that the color is brown. This coral was pink when I
bought it. Here is a photo taken early October last year. I wonder
if this is related to the 2 dying corals. I hope I can still save
these 2 corals. Your advice is greatly appreciated. Sammy <...
I'd be removing the Cuke, and reading here:
http://wetwebmedia.com/cnidcompppt.htm, the linked files above...
and re Anemone Compatibility period... You have iatrogenic
(self-caused) troubles here. Bob Fenner> |
|
 
|
SPS Bleaching 9/15/05 Hi, I have a situation that
I believe has me on the borderline of significant problems and wanted
to get your opinions on what I can do to get back into the
clear. I have read several of the other messages with
respect to coral bleaching and think that I have some ideas of what I
may need to do based on feedback that you offered. I just
want to make sure that I do the right thing, so here
goes. My problem is that I have a SPS bleaching problem, but
my tank conditions seem to be within (or at least bordering) what you
have shown as acceptable conditions. <Often, the
problematic conditions that lead to bleaching or dying are not things
we measure for.> First the specifics; the tank is a 180 gal system
with about 140 lbs of live rock with a 65 gallon sump which services
both the 180 and a 70 gallon refugium on a reverse lighting schedule
with a 5 inch deep sand bed. The sump also has about 30-40
lbs of live rock in it from a prior rearrangement where I didn't
have room for it in the main tank. The total volume is
around 300 gallons give or take. The system has been in
operation for four years now and I decided six months ago to try to
keep SPS corals. Although the system has a moderate fish
load (yellow tang, 10 yellow tail damsels, Midas blenny, Lawnmower
blenny, 6-line wrasse, and a pair of percula clowns which are fed daily
with a variety of items like Cyclop eeze, formula one and two and
Spirulina. I also dose DT's on an every other day basis
adding about a tablespoon at a time. I run a pair of Tunze
skimmers and the water seems to stay clear with no noticeable
yellowing. I also have pretty good coralline algae growth
and minimal hair algae in the tank- just a little rust colored algae on
the back wall and some Valonia in small controllable
patches. Finally, the tank has 3 clams which have been doing
great for years- a 14 inch Maxima and two Croceas that are about 5
inches at the long points. To accommodate my attempt at
keeping SPS corals, the tank has a pair of Tunze 6100 stream pumps and
I have recently added 3 400w 20K MH bulbs to the 4 110w VHO's that
were over the tank. The lights are turned on and then off
stepwise during the day with the halides on for about 6 hours during
the middle of the day and a 12 hour total lighting
schedule. I have a calcium reactor as well as a Kalkreactor
that run continuously. The Kalkreactor consumes about a tablespoon of
powder every 2-3 days. I also add Lugol's solution at
the rate of about 1 ml. once a week and add a teaspoon of buffer 3
times a week. All additions are to the sump. Water
evaporation replacement is done by overflow from the Kalkreactor and
untreated water. <All sounds good, but that is a LOT of
light! Let's see what else is going on...> Test
results on the tank are as follows: pH typically is 8.1-8.4 dependent
on time of day, nitrates are zero, alkalinity is 10-11, and Ca++ is
around 420 (hard to tell with a LaMotte test kit where the purple/blue
transition is exactly). I live in Virginia so the tank temp stays
around 79-80 in the summer and I drop it to 78 in the winter- it is
held constant with a chiller. <All sounds very good.> I have
added about 10 frags and 4 colonies total- mostly Acropora SPS with a
Turbinaria LPS and a Pavona of which half are doing great and growing
while half of the Acroporas are bleaching. All of the
additions were treated with tincture of iodine and then a tank water
wash prior to being placed in the main tank. One of the
bleaching specimens is a 3 inch diameter blue SPS colony that was added
just two weeks ago. Another is a tan colored Staghorn frag that has
been in the tank for 5-6 months had grown to at least triple its
original 1 inch size prior to beginning bleaching this last
week. The tan Staghorn stayed the same color its entire stay
and about half is bleached as of today. Both of these corals are at
about the midway point in the tank which would have them about 14-15
inches from the MH bulbs and 5-6 inches below the water
surface. I have one SPS frag that turned from fluorescent
yellow to white about 4 months ago, but it has not shown algae growth
so I assume it is still alive. Finally, I had two other fluorescent SPS
frags which did seem to lose some tissue but parts were clinging to
life so I did not remove them. I'm not certain what RTN
looks like, but I don't see any tissue falling off these three
currently bleaching or already bleached corals or any slime being
expelled. I am concerned that I might have something
spreading and that my other corals that seem to be doing fine might
catch something? I also am at a loss as to exactly
what steps I should take to get whatever is going on back under
control- help! Thanks for any information you can give to me
that will help me figure this out or anything you can do point out
problem areas with my reefkeeping methods. Eric Black
<The addition of new colonies often leads to RTN and/or bleaching in
established colonies. Colonies under stress can produce chemicals that
produce extreme reactions in other corals. If the corals are
really bleaching (living tissue but white, usually from the tips down),
I still suspect light. If the corals are suffering from RTN
(tissue actually dying, often leaving lacy black tissue remnants,
usually from the bottom up), it is likely due to the addition of new,
stressed colonies. Bleaching is easier to deal with...
simply reduce the amount of light and slowly re-acclimate the corals to
it. RTN is trickier. It is probably best to
remove the offending colonies to a quarantine tank until they are over
the stress of shipping. Water changes and carbon will help
remove chemical messengers from the tank water. Good luck!
AdamC.>
| Turbinaria coral strange behavior 2/27/05 I
have been searching the internet for an idea of what is happening
to my Turbinaria... I have had it for a year, and it has been doing
fantastically until last week when it started to develop this
bubble. I did have to move it very slightly closer to the lights
recently, and nearer my branching anchor. Is this polyp
bailout? <it definitely does look like polyp bailout...
how ironic too, I use a pic similar to this in one of my
presentations describing how light shock or aggression from a
nearby coral (like your VERY noxious/aggressive hammer Euphyllia)
can cause this> Could it be getting stung by my anchor?
<easily so at night with modified sweeper tentacles on Euphyllia
that can reach 10"> I called my LFS and they had no idea
what it could be and suggested I dip it. <yikes! no...
please don't stress the coral any more... the LFS is mistaken
here> I appreciate any help you can offer. Great site, and
thanks! Kevan <best regards, Anthony><<To add my dos
centavos here... DO move one or the other of these colonies.
RMF>> |
|

|
Coral Aggression: Galaxea 1/7/04 Brant here again,
<cheers> I really appreciate having such an informative
site. I wanted to mention in reference to my last
e-mail about white band that I also got a Galaxea at the
same time. I placed it on the top of a rock in
the center of the aquarium with some distance between
corals. <grumble, grumble... would rather have heard it
was placed properly in a QT tank first for 4+ weeks. We might not be
having this exchange if so <G>> The Stylophora is
only 3-4 inches away and is somewhat 'downstream' from the
Galaxea. <Yikes! The Stylo is soon to be Galaxy
coral food> I've read a lot about sweeper tentacles
<eventually 10" long from Galaxea... they are one of the
worst> and was wondering if this had anything to do with
my Stylophora problem. <very easily so> My salt level
is low also, at about 1.019. <do get this up to
1.023-1.025 for corals> Besides the Stylophora problem, I was
wondering if I could/should place my Galaxea directly on the
floor of the tank at the farthest distance from everything
else? <perhaps... they are one of the most aggressive
corals in the trade> Your help is greatly appreciated. Sincerely,
Chris Brant <best of luck, Anthony>
Coral and anemone follow-up Hello Everyone: <Cheers, my
friend> Would like to give a special thanks to Anthony for his
advise regarding the tube anemone. <my pleasure> I
reluctantly removed it and couldn't believe how the rest of the
coral has responded. <they are indeed hostile>
Everyone is fully open and enjoying their meals per Anthony's
instructions. Even the candy coral seems especially happy
and has remarkably bounced back, <great to hear!> although I
couldn't find the brand name frozen food he recommended, I bought
Hikari's brand of Zooplankton and Mysis Shrimp (hope this is
acceptable). <no worries if the protein is comparable
(over 60%?)> Everyone seems to be eating just fine because they are
obviously very happy. Have been feeding them 5:00 AM when
their feeding tentacles are out. I do have a concern regarding a lime
green feather duster (with a soft tube). I'm having
trouble with bubble Caulerpa sprouting on it. Apparently
some time back it must have seeded itself everywhere.
<bummer> I have tried pulling it off the tube but it seems to
stress the tube itself. Also, there is a thick dark velvety
red algae growing on the last inch of the tube that seems to be getting
thicker. I've tried to scratch it off with my finger,
but it appears to be very dense. <do try a peaceful
grazing urchin like a Tuxedo sp (Mespilia)> The rest of the tube is
fine since I have it buried in the sand. So far it
doesn't seem to have bothered the feather duster. Should
there be concern? <little> Am also concerned about
roots from the Caulerpa growing inside the tube and bothering the
little guy. <agreed... remove when possible> Everyone at WWM is
just great, thanks for all the professional assistance. <our great
pleasure> May the force be with you. <it is... I had Mexican food
for dinner. Thanks for noticing. Anthony>
|
|

