|
FAQs about Caryophyllid Coral Disease Diagnosis
Related Articles:
Coral Pests and
Disease; pests, predators, diseases and conditions by Sara
Mavinkurve, Caryophyllid
Corals, Elegance
Coral,
FAQs on Euphylliid Disease:
Caryophyllid Disease 1, Caryophyllid Disease 2, Caryophyllid Disease 3, Caryophyllid Disease 4, Caryophyllid Disease 5, Caryophyllid Disease 6, Caryophyllid Disease 7, Euphylliid Health 8, Euphylliid Health 9, Euphylliid Health 10,
Euphylliid
Health 11, Euphylliid Health 12,
Euphylliid Health 13,
Euphylliid Health 14,
& Elegance Coral Disease/Pests,
FAQs on Euphylliid Disease by Category:
Environmental (Pollution/Poisoning, Lighting...),
Nutritional, Social (Allelopathy),
Trauma,
Pathogenic (Infectious, Parasitic, Viral)
Predatory/Pest, Treatments
FAQs on Stony Coral Disease: Stony Coral Disease 1, Stony Coral Disease 2, Stony Coral Disease 3, Stony Coral Disease 4, Stony Coral Disease 5, Stony Coral Disease 6, Stony Coral Disease 7, Stony Coral Disease 8, Stony Coral Disease 9, Stony Coral Disease 10, Stony Coral Disease 11, Stony Coral Disease
12, Stony Coral Disease 13,
Stony Coral Disease 14,
Stony Coral Disease 15, Stony Coral
Disease ,
FAQs on Stony Coral Disease by Category: Diagnosing:
Environmental (Pollution/Poisoning, Lighting...),
Nutritional, Social (Allelopathy),
Trauma,
Pathogenic (Infectious, Parasitic, Viral)
Predatory/Pest,
Treatments
FAQs on Stony Coral Disease by Family: Acroporid Disease, Acroporid Disease 2, Acroporid Disease 3, Acroporid Disease 4...,
Caryophyllid Disease 2..., Elegance Coral Disease/Pests, Dendrophylliid Disease, Faviid Disease, Faviid Disease 2, Fungiid Disease, Mussid Disease, Mussid Health 2, Poritid Health, Trachyphylliid Disease, Trachyphyllia Disease 2,
FAQs on Stony Coral Disease by Type: Brown Jelly Disease, RTN,
|

|
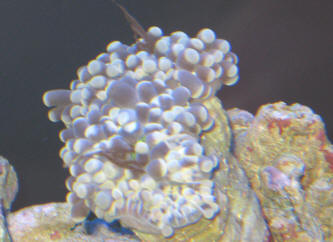
Torch coral showing some skeleton
4/6/20
Hi, firstly i hope everyone at WetWeb and their families are ok in these
troubled times.
<Hi Toby, mostly fine, thank you.>
Now to my question to which i cannot really see an answer to on the site
or elsewhere. I have had a torch coral for about 3 months now and it has
looked healthy. Very recently i have spotted when its tentacles are
moving in the current that a part of one side, say 10%, of the coral has
peeled away from the skeleton beneath.
<I see>
The torch coral overall seems healthy, no brown jelly or shrunken
tentacles
<This is a good sign>
the area that is no longer fixed to the skeleton is no different to the
rest of the torch. The skeleton beneath is white and clean. My tank
parameters are stable and other corals are in good health.
<Please send the exact numbers, “stable” is a relative term.>
I suspect i have had this torch in too vigorous a flow which has lifted
one edge off the skeleton.
<Yes, could be just the water flow stressing it out.>
I have relocated the torch to a gentler flow spot where it seems just as
happy.
<Good move>
There was nothing to sting this torch and looking at the skeleton i
don't think this is anything to do with reproductive splitting.
<Me neither>
I have read one or two things suggesting the absence of certain trace
elements can cause heads to detach from skeletons
<Yes, low levels of certain elements like strontium and magnesium can
cause this.>
but my gut feeling is that too strong a flow is the culprit. Do i need
to do anything to help the torch? In the lower flow the detached 10%
area does not lift as it did in the higher flow. Will it reattach? It
certainly should not rip away further if current was doing this. I would
appreciate any advice,
thanks for your time,
Toby
<I suggest checking the mentioned elements just to discard the
possibility that they are below the required levels, other than that, I
would leave the torch on the new location and watch it closely for the
next few days. Cheers. Wil.>
|
Torch coral help? 10/27/16
Hello WWM crew,
I recently purchased a Torch Coral and just now noticed some skeleton
exposed at night. So I flipped the lights on and did a visual inspection. I
can't see any brown decay but I'm still worried about Brown Jelly Disease.
The head in question is the one on the far right.
My levels are:
Mg-1300ppm
Alk-7.3dkh
Phos-0ppm
<Ahh; all life needs "some" soluble/useful HPO4... and NO3. See, as
in read on WWM re. MANY people have been misled by chemical filtrant sellers
on this issue. Your system may simply be too sterile chemically to support
this life>
Ca-440
Ph-8.2
Temp-26 Celsius
Salinity-1.025
Gallons-75
Current stock:
Diamond Watchman
Barnacle Blenny
Snails (turbo tophats and Nassarius)
Hermits (dwarf zebra, electric blue)
Leather (toadstool)
Photosynthetic gorgonian
Mushroom
<Other usual "causes" include (other) environmental issues... too
much/little of something necessary (physical, chemical, biological);
predation/harassment (perhaps the hermits here), Allelopathy (maybe the
Sarcophyton, Shrooms...>
It has just been only 2 days since I bought the Torch but any help
would be much appreciated.
<Oh, or just general stress... moving is VERY stressful. See WWM re the use
of iodide-ate (I'd triple dose the system, then three days later, double
dose), the possible use/administration of simple sugar. Write back after
reading if your course of action, and rationale aren't clear. Bob Fenner>
Thanks!
Kellan
|
 |
Euphyllias... hlth.
6/13/16
Thank you. I notice something odd. The hammers in my 265 for the most
part are doing well. Their heads get larger and the babies double their
sizes in about 3 days. But there are few heads on the side that
keep dying like the big heads are competing causing their demise?
<Possible... I encourage you to review the FAQs archived on WWM re this
family's compatibility and health. Solutions frequently sought include
the use of chemical filtrants, ozone.... moving the considered
offender/s,
offendees... fragging... Bob Fenner>
|
Frogspawn issues
I noticed on Euphylliid health on your website about pests and parasites and
different solutions. I have not noticed any hitchhikers. The thing I
noticed that my nitrates are high
<How high is high?>
and that I changed over to a max gyre wave maker and ever since that I noticed
the frogspawn respond differently in that it has not all bloom as nice as
before. The wave is not so high to put a lot of current on the frogspawn section
that is being effected. These are a few more different pics that might
be able to help with my issue.
<See some dead heads... long since>
Also I noticed last night a small piece of frogspawn tips on the sand bed.
<Happens; a type of "bail out".... specimen-saving behavior.... escape mechanism
from poor conditions locally. You need to find out what these "poor cond.s"
are... Too little of something/s, too much of...?>
Could it be a fish picking at it.
<Doubtful; Euphylliids/Caryophylliids aren't very palatable... too stinging when
healthy>
I have a coral beauty, yellow tang, blue throat trigger bi color blenny and a
melanarus wrasse. Can I send you a video of my tank and maybe that would be more
helpful?
<You can send a link to the video you post on the Net; but again;
something/s wrong w/ the environment here (the list of possibilities is very
long).... When, where in doubt, large changes.... water changes, replacing
substrate, a good deal of new live rock.... best perhaps moving the colony to
some place new that's established (another system)....>
As I said before this frogspawn is about 20 yrs old. It is or was pretty hearty.
Thanks again for your response. I much appreciate it
<I'd be reviewing the environmental diseases of Scleractinians, the
Families therein; posted on WWM.
Bob Fenner>
Frogspawn issue
Oh yeah I forgot. I also use revive coral cleaner
<Don't know much about this>
when I introduce any new corals which everything else is doing real good other
than a section of the frogspawn. Also how would I administer iodide-ate
and hexose sugars?
<See/Read on WWM re... the search tool....>
Do I take the coral out or dose the tank?
<The latter likely>
Thanks,
Vasilios
<Welcome. BobF>
Re: Frogspawn issues
Thanks for the quick response
<Ah, welcome>
|
 |
|
Re: Frogspawn issues
Hello Mr. fennel. I forgot to mention that I have a purple lobster also a blue
and orange Linckia and yellow mesh starfish. Would they be an issue with the
frogspawn?
<Mmm; not likely. They might be crawling over the colony, but shouldn't result
in the dead areas shown in your pix>
I have had them for a couple of years without problems
Thanks,
Vasilios
Re: Frogspawn issues
Nitrates is 75
<MUCH too high. For reef systems you want to keep [NO3] under 10 ppm maximum.
SEE/READ on WWM re nitrate control.
http://wetwebmedia.com/nitratesmar.htm
and the linked files above. Bob Fenner>
Re: Frogspawn issues 7/18/15
Hi Mr. fenner, I truly thank you for your experienced input.
I went to the link, very informative.
<Welcome Vasilios>
I have a blue Linckia which is doing good but an orange Linckia is shrinking in
size and a leg seems a little shredded. also my yellow mesh starfish is doing
good. can any of these be a culprit in eating some frogspawn even though they
have been in the tank for at least 2 yrs?
<Again; like the other invertebrates last listed, not really. These Stars can
disturb other organisms by crawling over them, but rarely do deaths result>
i also have a purple lobster. I have never before seen any of these do any harm.
Just wondering I will definitely make a big water change soon and cut down on
the food. I have have been giving them flakes twice every other day and then 2
cubes
of mysis every other day. I will only feed them flake once every other day and 1
cube of mysis every other day. Would that be better?
<Possibly... I'd keep reading... a DSB, macro-algae culture and more are better
possibilities. BobF>
Re: Frogspawn issues 7/18/15
thanks i will keep reading
Vasilios
<Good>
Re: Frogspawn issues 7/18/15
what's a DSB?
<The reading>
|
|
Frogspawn; hlth. diag. 7/14/15
I am trying to figure out why some of the frogspawn is not blooming. I
have not changed anything in my tank. The frogspawn is around 20 yrs old. What
can I do to remedy this? Help
<Mmm; well; same ole checklist of possible predators, deficiency syndromes
(alkalinity, P, K)... usual shake n' bake possibility of trying to flush out
pest/s.... administration of iodide-ate and hexose sugar/s... Have you read on
WWM re Euphylliid health?
Bob Fenner>
|
 |
|
Hammer Coral Questions 4/25/15
Hello Bob,
<Hey Vinh>
It's Vinh again hoping to pick your brain. I have these two corals that I'm
concerned about. They're both hammers. One is the common green branching hammer,
which seems to have turned snow white on the stalk, but the
head seems fine. Is this from a lack of light? I do feed weekly.
<Might be from just being close to the other... I'd give them another few inches
space>
I read through your FAQs section and did find a similar case, but wasn't sure if
this case was referring to the tentacles or the stalk:
""Bleached hammer coral Hi I have a 29 gallon mini reef that has a branched
hammer coral that turned white a few months ago.
<likely from a attrition (starvation from lack of light and/or lack of target
feeding) or salinity/temperature shock. The latter can occur and not effect all
coral... different tolerances with each. Do you recall a sudden increase in heat
or a lapse in evap top off followed by the dumping of a sudden large amount of
freshwater in to compensate? If not... the coral was simply starving... very
common. Many poorly lit or underfed coral can go 6-12 months before finally
waning noticeably> It doesn't open as large as before but otherwise it seems
fine. It has been like this at least 6 months. <Yikes>
The other one is an orange wall hammer, the left side is turning translucent. Is
that a sign of bleaching, possibly from a lack of light as well?
<Possibly; but could be due to many other causes... a nutritional deficiency,
prior stress...>""
I have a Maxspect Razor 16K LED 27". It's 16" above the surface of the water.
The quarantine tank is 12" deep. Do you think I need to increase my lighting
intensity?
<Worth trying; or moving the specimen/colony up closer to the light. Do you have
access to a PAR, PUR meter? Maybe the LFS can lend you one>
Below is my lighting schedule:
Time Intensity
8:00 0%W
1%B
12:00 6%W
9%B
14:00 12%W
35%B
15:00 29%W
42%B
21:30 0%W
1%B
Here are my latest parameters:
Salinity: 1.023
<I'd raise this to 1.025-6>
PH: 8.23
Temperature: 77 degrees
Calcium: 450
Magnesium: 1500 (on the high side so I've since started lowering the
dosage
Alk: 9.1
Phosphate: .1
Nitrate: 0 (I've cut back on the carbon)
<Need some measurable NO3; this could be "it">
<Bob Fenner>
|
 |
|
Zoox expulsion and recession 6/11/13
Hello!
I have a 1 year old 120 gallon reef tank. Only 3 fish, a couple of
gobies and a Royal Gramma. Lights are 2*175 Watt MHs with 2*54 Watt T5s.
Temperature is typically 78 degrees, Nitrates under 5 ppm, phosphates
haven’t measured. There is a little nuisance algae but not much.
Alkalinity and calcium have been a little low, with 6 kH, and 360 ppm
respectively.
<What re Mg?>
Last week I started adding alkalinity to get it up, and added Kalk to my
ATO. I do a 5-10% water change with RO water/salt every 1-2 weeks. There
are 2 Koralia 1400 pumps plus the return, and circulation seems more
than adequate. Corals are all LPS, 4 Euphyllia, 1 Trachy, 1 lobo, 1
Caulastrea, a couple Favia/favites. I have a couple of new Monti Caps in
quarantine.
About 6 months ago when I added 2 Euphyllia glabrescens, one Indo and
one Aussie, I noticed them ejecting what I’m positive was
Zooxanthellae. It was dark brown, thick and stringy. Though I feed my
corals weekly with Mysis, there was too much for it to be waste and it
was too dark. I shrugged this event off as unimportant, because the
corals seemed otherwise fine.
<Mmm, and the reason for this expulsion? Heat, change in lighting,
addition of Cnidarians...?>
About a month ago I was away on vacation, and when I came back, one of
the other Euphyllias, an Ancora was quite bleached. I’m guessing it may
have been a heat spike since the weather got warmer, and my building had
yet to switch over to air-conditioning from heating (it’s an older
building). Since then I noticed that the Ancora would shrivel up
whenever the metal halides would turn on. I moved it down, and reduced
my metal halide photoperiod from 7 hours down to 5 (the 2 T5s are on for
12 hours).
<Good>
It still would react negatively to the metal halides, even thought the
175W 14K Hamilton's I have are suppose to be a pretty low PAR bulb.
<I'd be measuring PAR/PUR at their depth>
I kept reducing halides duration until I found with a 2 hour photoperiod
(T5 still at 12 hours) the Hammer began to look better, more full. It
continues to look good, though still bleached, as long as I keep the
halide photoperiod short.
This brings me to the last couple of days. I noticed that one of the
torches shows some recession. Not to where the septa are exposed yet,
but tissue has risen pretty close to where the septa might start, and
some white skeleton at the “neck” of the polyp is exposed. The polyps
are inflating normally otherwise. Tonight I noticed the torches again
ejecting large amounts of Zooxanthellae towards the end of the
day, as the polyps were starting to close up. Their color hasn’t
changed. The part I find surprising is that I thought this usually
happens with excess light, but I only have a 2 hour metal halide
photoperiod now!?
<Along w/ whatever else is going on here, this is evidently too much>
Clearly something is up with 3 of my Euphyllias. One bleached, one
receding and ejecting something, and a 3rd only ejecting something. The
4th, what I think is a E. yaeyamaensis, looks perfect, inflating nice
and huge, and with zero recession and zero expulsion of anything but
normal waste. The other corals also look good.
Is this perhaps not light related?
<Something else "is going on", but what? Perhaps another unseen invasive
organism... could be a (micro) nutrient shortage...>
Is my photoperiod too short now? Can alkalinity of 6 be the source of the
problem? Can you offer any wisdom or ideas as I’m not sure how to
proceed?
<Yes... I'd try the product Zeospur 2 here, as a supplement... Do read a
bit re others impressions... on the Net>
Thanks so much,
Dave
<Welcome. Bob Fenner>
|
 |
Fw: Zoox expulsion and recession
6/11/13
Hello WWM crew! I sent this email/question last night, but thought I should
have added another picture for clarity. One is of the Aussie Torch expelling
what I think is Zooxanthellae, and the other I just added, is of the
recession of the other Torch coral. Sorry for any confusion this may cause.
Dave
<Mmm, thanks for the additional pic... am concerned re the red spot at the
base of the colony... is this BGA? If so, either it, or the conditions
allowing for it may be the root of the issue here. Please search, read on
WWM re Cyanobacteria. Bob Fenner> |

 crop
crop |
|
Re: Zoox expulsion and recession
6/12/13
Hi Bob,
Thanks for getting back to me. You asked if it was BGA on the skeleton.
I stuck my hand in and gave it a scrape. It feels velvety, but did not
come off.
<... likely IS BGA then... can you look at a sample under a 'scope?>
It doesn't seem to be coralline, and doesn't seem to be Cyano. I think
that spot has been there since I got the coral about 5-6 months ago. I
actually did have some persistent Cyano on the substrate a couple months
back, but after some suctioning with water changes it seems to be mostly
gone other than a small spot near my Trachyphyllia that comes and goes.
I want to keep my halides at 2 hours a day for the next while, with the
two 54W T5s at 12 hours, since my bleached hammer seems to prefer this.
Is this a problem for my other corals, for the next month or two?
<Not likely; no... most aquarists over and mis-light their
photosynthates... think on this; the sun is only directly overhead a bit
every non-cloudy day... Not much, often. And photosynthetic organisms
are very capable of adaptation>
Also, could the
slightly low alkalinity and calcium be the cause of my problems?
<More likely an imbalance issue; most often w/ Magnesium... when in
doubt, test, do water changes>
I will check out the product you named. Thanks again!
Thank you,
Dave
<Welcome. Bob Fenner>
|
|
Re: Torch Coral Receding (might have been sent twice)
8/16/12
Crew,
<Art>
I appreciate all your advice and I apologize for the long delay in letting
you know what happened to my Copperband. Unfortunately, though it did begin
eating, about 2-3 days after my last email it died.
<Sorry to read/realize>
It appears, as it so often does in this hobby, that my trouble is not over.
This is the fastest growing algae I have ever seen and after I remove it,
largely via siphoning it off at water changes, it comes back within a week.
<Need to "fight" in other ways... competition, denial of nutrient/s,
predation...>
My system has low nitrates, less than 5 ppm, and zero phosphates, or so my
test kit says, but I couldn't tell the difference between the lowest three
colors anyway so maybe I'm reading it wrong. I have read that if there
is enough algae in the tank it will utilize nitrates and phosphates faster
than they can accumulate so tests don't register.
<This can be so>
I believe from what I have read that this is likely caused by my rocks
leaching phosphate into the tank, as about 1/2 of the 100+ lbs were dry base
rock when I put it in (2.5 months ago). So they were not fully
developed (for lack of a better word). I did make sure the tank was
fully cycled before putting in fish. That being said I had to take all
of my corals from this tank and move them to my 34 gal Solana so that they
were not being choked by the algae.
<Ok>
Now the new problem I am having is that since the end of July my torch coral
has been getting smaller and smaller. It has about 5-6 heads and is
maybe a 4 inch circle. I feed my corals in this tank Azoox Coral Food,
Mysis shrimp, and the occasional Spectrum Pellet that falls on them.
Everything looks pretty good except the Torch and my Duncan, it was being
harassed by clownfish and sexy shrimp in the bigger tank so it looks bad but
I'm hoping in the shady spot in the Solana a few of the heads will be saved
and I can frag off any dead parts.
So I have a few questions:
1) Is the torch coral not getting enough light ( about 150 par) or not
enough?
<This lighting, PAR value is fine (anything more than 100 would be)>
The larger tank has t5's and LED supplements and the smaller tank has
straight LEDs.
2) Is it improper flow? The area is relatively calm and my coral looks
no different than the ones in the fish store when it was extended?
<Not likely a/the issue>
3) Is the chemical warfare amongst my corals killing off the torch because
its more sensitive?
<Could be... but the new-ness of the system, it's lack of stability overall
is likely much more cause>
4) If so, how can I fix or temporarily mitigate the fighting?
<Read here:
http://www.wetwebmedia.com/CorlCompArt.htm
and the linked files above... Best to "start over" in stocking if
practical... isolate (quarantine) most/more mal-affected, reintroduce over
time>
(Other than the GAC and water changes (about 5 gallons or so weekly)) I
only need to leave the larger tank coral-less for another couple months
until the dry rock stops leaching phosphates.
5) Are my nitrates too low in the 34 gal (don't register on API kit)?
<Not too low, no>
6) Should I just move it back to the 75 gal where it will be in a much
bigger area away from all the other corals?
<Yes>
(Again, the problem here is that the super fast algae chokes out my corals
in a matter of a week or two meaning I have to take them out and clean them
routinely).
<You NEED to solve this algal issue... Read here:
http://www.wetwebmedia.com/algaeconMar.htm
and the linked files...>
I have a couple Zoas and palys in the 75 to see if they survive and they
seem to do O.K. (i.e. they multiple and have good color) as long as the
algae doesn't get to them. Would that mean that the Torch stands
a better chance in there?
<Considering the algal issue, the present condition in the smaller system,
more likely yes>
7) Somewhat unrelated to the torch, have you ever heard of algae growing
like this, it doesn't even bother to form complex structures (i.e. no
identifiable strands, veins, roots) its like a brown fluffy version of red
slime algae?
<Oh yes... unfortunately many times, places.>
None of my LFS people had any suggestions other than to keep doing what I'm
doing.
<Mmm, Read on WWM re>
Sorry for so many questions and information all in the same email but
everything seems related and I'm not sure where to separate them, and to be
honest I have only been able to keep Euphyllia for 11 months tops
(frogspawn), most of them are much shorter lived so this is a problem far
exceeding the current issues.
Yours truly,
Arthur
p.s. If you think that my belief about the rocks leaching phosphate for the
first several months is wrong let me know because if not I have another
problem.
<Could be a contributor... easy to melt a bit (organic or not acid...
CH3COOH, HCl, test for HPO4) and test for>
p.p.s. I attached pictures to the bottom of the email. They should be
a total of less than 500 kb. The top (or first from the left depending
on how you receive this) is a picture when I first moved it. The other
two were taken a few days ago and shows both the same angle as the first
picture and a side shot of the coral (will have to use your imagination for
the side shot of the first picture sorry).
Solana Reef Tanks stats
78F
470 ppm ca
<Too high... need to review where you're boosting this and slow down, stop
perhaps>
(having trouble keeping this down and Alk up so this is as close as I can
get using 2 part, hopefully the corals will catch up)
8-9 dKH alk
1350 ppm Mag (but for past month it has been dropping from 1500 ppm as I put
additives into the tank)
35 ppt salinity (1.026 sg)
pH is between 8.2-8.6 (Usually between 8.2 and 8.3 but last week Wednesday
the meter read 8.6 so I went to calibrate it and it needed no adjustment but
the next day, and since, it has been within normal range)
I use:
Oceanic salt mixed in separate mixing barrels for up to 2 weeks before use
and I change weekly about 5 gallons.
RO/DI water with a 0 TDS reading from the meter
Purigen (couple table spoons)
GAC (changed weekly)
Phosban (Changed Monthly)
<I'd ditch this for now>
Equipment:
AI Sol Blue
Vortech mp10 in Nutrient Export EcoSmart Mode
Tunze Nano 9002 (Just got it for the Solana a few weeks ago and just got it
dialed in a few days ago)
Tank Tenants
Torch Coral
Small Cristata Euphyllia (I know there might be an issue with allelopathy
between the Euphyllias but this coral is about 1/4 the torch and they are
not near each other)
Florida Ricordea (one orange one green)
Metallic Green Mushroom
Assorted Zoas and Palys
2 small (2X1" Acans) - One of each species
12 Cerith Snails
Duncan Coral with 7-12 Heads
1 Fire-fish
75 gal Tank
78F
0 Ammonia, Nitrite, < 5 ppm Nitrates
Don't measure Alk, pH, Ca, Mag, since hard corals were removed but at the
time they were pulled out:
Ca 450 ppm
Alk 9.3 DKH (steady)
pH 8.2
I use biopellets and a Octopus 200-xs skimmer
Tenants
Dragonet
Mystery Wrasse
Royal Gramma
Pair of Occ. Clowns
few Zoas and palys
Used to have butterfly fish but now that its dead I might remove biopellets
(or buy more fish)
<Bob Fenner>
|
 |
|
Re: Torch Coral Receding - 8/17/12
Dear Bob Fenner,
<Arthur>
Thanks for the quick reply. To clarify there are two tanks I am
talking about. The 34gal has been set up for over a year and was
supposed to be used for Zoanthids and a couple frags of stony corals.
It is the 75 which is new and having this algae problem and which was
where I put the corals from the 34 gal after I moved about 2 months ago
(the 34 came with and the 75 i set up in new house).
<Ah, I see>
I seem to be getting mixed signals regarding allelopathy. I was
always under the impression that Euphylliids were low in the chemical
warfare department
<Ah no... the genus ranks near the top... 8 or 9 on a scale of 10. See
WWM... the Caryophylliid Compatibility FAQs>
relying more on sweeper tentacles to compete. According to this
http://www.wetwebmedia.com/CorlCompArt.htm Euphylliids (is that the
proper plural form?)
<Mmm, yes... Veron's last work... he "elevated", created a new family
for this genus, others>
are inside the sweeper tentacle section but are not directly referenced
in the allelopathy section. However, most of the user questions
refer back to the articles on allelopathy, which I always thought was a
big problem with Shrooms, Ricordea, Yumas and Plate corals (in weakest
to strongest potency).
<Mmm, no; your order is fouled up... again, gone over and over on the
site>
You mentioned in my question that its not likely the main cause of my
coral's current predicament anyway.
<Is, but/and "brought on" by the challenges detailed/mentioned by you...
a typical "cascade event"... Read here:
http://www.wetwebmedia.com/toxictkendof.htm
Moving the corals from the 75 to the 34 was my way of keeping them in the
more stable system that has been set up and successful. I had planned on
waiting 5-6 months (thinking November/december) before reintroducing
corals into the 75. So for my understanding, are you suggesting
that I move the Torch back to the 75 which has only been set up for
about 3 months?
<Yes I would>
Maybe I go the other way and just put in the toughest corals for now
(scans and Zoas) and have just the torch in the 34 gal? I have no
other option for tanks with lights on them, my local fish store won't
hold anything long-term and I am all out of reef tanks.
So to address the rest of the issues with the algae in the 75. So
I put in some Chaeto into the sump from another tank to hopefully suck
up some of the nutrients to help limit what the brown algae gets.
<Please... read... >
It has been in the sump for about a week and its a nice dark green color
and seems to be growing at least a little. I currently perform
25-30 gallon changes weekly on the 75 to try to combat the algae and do
not run Phosban on this tank. I was thinking about switching
from Phosban to Phosguard by Seachem but I read that it is an aluminum
based absorbent.
Didn't iron based products replace them as the aluminum would irritate
the corals.
<Not in low concentration... and there are other/alternatives...>
As far as predation goes I have two turbo snails, 20 banded Trochus
snails, and half dozen Astrea and Cerith snails, oh and one Nerite
snail. As far as predation of the algae should I add more
snails?
<No>
I don't want to be two months down the road, fix my phosphate (maybe)
issues, and then have a bunch of starving snails. I was also
thinking a Starry Blenny and a Hectors or Jester Goby to help eat the
algae. If you have any suggestions please let me know. The
other thing I was thinking was to remove my yellow-eyed Kole tang
(purchased on your recommendation) from my 120 FOWLR and put it in the
75. However, I always feel chasing them around the tank is a lot
of stress so I may just leave it in there and get some other fish that
does similar work.
As far as my current progress goes as I missing anything else. I
don't want to toss the rocks so I guess I will have to wait but in the
mean time is there anything we haven't talked about that I can do for
the long run?
<... keep reading. B>
Also as far as testing phosphate goes the biggest problem I have is
telling the difference between the lowest levels my test kit gives out
(Salifert).
This seems to be a problem with all the test kits for phosphate that I
have been shown. I don't know if you are willing to endorse/comment on
an particular product over your site but I was looking into the Hannah
Phosphate Checker, the electronic ones. Do you have any personal
knowledge of these products, their error rates can be 10% which seems
awfully high and could potentially limit the usefulness of the digital
readout.
Yours truly,
Arthur
p.s. The eventual stocking list for the 75 is the current fish
(mystery wrasse, royal gramma, pair of clowns, and dragonet) plus pair
blood shrimp, starry blenny, small goby (hectors/jester), watchmen goby
and one larger fish, like a small tang or Butterflyfish. The total
volume is around 95 gallons and the tank is 48x18X18 is that enough
room?
|
|
Frogspawn issues 7/7/11
I have successfully kept a healthy reef tank for 3 years.
It's a 30 gallon mixed reef tank. The predominant corals are
Torch, Frogspawn, Hammer, and Acan corals.
<You're to be congratulated; it is hard to keep
Euphylliids together in such small volumes>
Parameters are triple checked and spot-on, however, in recent
months I have seen some die-off of my LPS.
<The Acanthastrea I take it you're referring to>
My theory was that the introduction of a new pump and the
increased flow it provided damaged my Frogspawn. The end result
has been a slow-motion polyp bailout, taking place over several
months. one head after another, until the entire coral dies.
<Mmm, more likely a negative interaction w/ another stony
coral here>
This bailout continues even today, as I just lost another head of
Frogspawn. As has been the case, the head is fully inflated but
just dangling off the skeleton by a thread.
On my other LPS, a wall hammer, I also am noticing a slight
receding of flesh, and it is not inflating as large as it once
did.
In short, none of my LPS are inflated as they used to be, and I
am seeing slow-motion polyp bailout.
<Something environmentally awry here>
After reading on your wonderful site, I suspect something else
might be occurring. I do have a few colonies of Zoanthids, some
pest green polyps, a few mushrooms, and Ricordea. I wonder if
what I am really experiencing is coral allelopathy?
<Possibly>
Especially since I have been trying to manually remove the
invasive green polyps with a pair of tweezers (a few polyps
culled every week or so.)
<This NEEDS to be done outside the system, the rock area
thoroughly washed, rinsed before being re-inserted in the
tank>
If coral allelopathy is indeed the case, I'd suspect I need
to remove the rocks with the offending soft corals, correct? Or,
could the attempted removal of the "pest" polyps be to
blame?
<Either, both>
I do run carbon, replaced every two weeks, I run a GFO
reactor,
<Why? The absence of nutrient could be the root cause here as
well...>
an Aqua C Remora Skimmer, and I do weekly water changes, and not
all LPS in the tank are hurting, which makes this even more
puzzling.
<Points more to allelopathy>
One last thing. I have noticed small, all white flatworms
gathering on some of my Ricordea. I wonder if they are doing
something odd to my LPS?
<Not likely; though there is a small potential here>
Parameters:
78.8 degrees F,
1.024 salinity,
<I'd raise/keep in the 1.025-6 range>
10 Alk.
8.1 Ph.
Thanks again for your wonderful web site. I really appreciate
your help.
I'm at a total loss at this point as to what is causing
this.
<Let's see your other mails. Bob Fenner>
Re: Frogspawn issues 7/7/11
I apologize for the second email. I neglected to mention the
large colony of Green Star Polyps that has begun growing
rapidly.
Thanks again.
Burt
<Mmm, you did mention. BobF>
Re: Frogspawn issues 7/7/11
Here is a photo:
http://i.imgur.com/6uenD.jpg
<Ahh, very nice!>
The two frogspawn on each side of the pest green polyps have had
slow-motion bailout.
<Well... if you had another system up and going, I might
remove the potential colonies one by one... see if this makes a
difference... AS all have lived together for such a long while, I
am discounting much of the mal-interaction... Perhaps some aspect
of your water quality has drifted...
I would ditch the GFO reactor unless you have a very compelling
reason for using it... Look into measuring RedOx, doing what you
can (simply) to improve this measure... See WWM re. Bob
Fenner>
|
|

|
|
Re: Frogspawn issues
7/8/11
Thank you for your reply. I am working to address your points.
One last thing I should mention; I have never actively fed the
Frogspawn or Hammer.
Could slow starvation also be a possibility?
<Mmm, yes... including a dearth of soluble phosphate from the
GFO use.
BobF>
|
Torch coral declining 6/3/08 Hello crew!
<Hello Dan! Benjamin here tonight.> I have been reading your site
for quite a while, and also have a copy of "The Conscientious
Marine Aquarist", which I really enjoy. <Great book to have>
Let me give a bit of background about my tank before I tell you about
my recent problem. I have a 14 gallon Oceanic BioCube in my office,
which I started last fall, so it's been in operation for about 8-9
months now. I've been keeping a log of everything I've done
with the tank. <Bravo! Tank logs are great for helping solve
problems, and for personal scientific inquiry. I wish more people kept
them.> I started with about 6 pounds of live rock, added a cleanup
crew of 4 hermit crabs, 4 turbo snails, and 4 sand-sifting snails, and
then slowly added fish (there are currently two Percula Clowns and a
Banggai Cardinal, and I don't plan on adding any more). These fish
have been doing great ever since I added them. I feed them a
combination of dry pellet food (which the clown fish devour but the
Banggai ignores), and frozen Mysis shrimp (which all three fish love).
I try to do water changes at least once every two weeks and sometimes
more frequently. I change about a gallon at a time. I also remove
excess algae. I've been fighting a never-ending battle with a
fast-growing strain of Caulerpa (I like the fronds but if I leave it
alone, it quickly takes over the tank!). <Sounds good'¦>
The beginning of last November I added a colony of purple Zoanthus,
which have been doing fine. About 10 days later I added a torch coral
colony. This coral has been thriving, and happily splitting off new
branches, until about a month ago. I started noticing that two of the
heads nearest the bottom (the coral sticks out of a live rock at about
a 45 degree angle) had retracted. These two heads eventually shriveled
up and died, leaving just the skeleton. I thought it might be because
there wasn't enough light down there, and since the other heads all
seemed fine I didn't worry too much about it. However, as time has
passed more and more of the heads have started to shrivel up and die
off, and the other heads haven't been as extended as they used to.
This coral used to be very beautiful and fully extended during the day.
Now it extends less than half of the distance, on average, that it used
to. I've read on your site about brown jelly disease, but none of
the heads have shown any brown film. They simply shrivel up and die.
<Hmm'¦wasting away. Troubling.> I've started
troubleshooting what might be the cause. The first thing is water
quality. <This is also what first comes to my mind'¦perhaps
Ca+/ Alk problems?> One thing that I've noticed with this tank
is that the alkalinity drops pretty quickly if I'm not vigilant. I
let it get down to 6 degrees KH at one point! I usually am able to keep
it around 8 degrees KH. The LFS (a really nice place) where I bought
the torch suggested I slowly try to raise the KH to around 11-12, so
I'm working on doing that. My calcium is about 420 ppm and
doesn't deplete as quickly as the alkalinity. <Ca is perhaps a
bit high, which can explain the low Alk. But this shouldn't be
causing your problem, as far as I know.> Throughout the life of this
tank, I've always read 0 for ammonia, nitrite, and nitrate, and pH
has been very stable at 8.3. <And this is true also at present? Just
want to double check, because poor nitrogenous waste disposal/ build up
would be another possible cause we can check off the list if you've
only just tested your water.> Another thing that I thought might be
the cause is lack of food. I've heard just recently that feeding
these corals Mysis shrimp is a good supplement to what they get through
photosynthesis. So, the last few days I've started target feeding
the individual heads. The ones that are most retracted ignore the food,
but the ones that are a bit extended do appear to grab some of the
shrimp and eat them. <This could sure play a part. Starvation is a
common cause of coral die-off in an aquarium'¦most notably in
situations where no supplemental protein is provided for tissue growth,
or where there is insufficient light.> Another possible issue is
water flow. There is currently moderate flow in the tank due to the
filtration system, and the polyps wave gently in the flow. <Sounds
fine to me, unless it is laminar current.> Finally, I've noticed
that two of my hermit crabs have started crawling around the heads and
nibbling at the edges of them. At first I thought they were just eating
the dead, shriveled polyps, but today I noticed them nibbling at the
edges of the live heads. Again, the clown fish and the Banggai Cardinal
pretty much leave the coral alone. I also noticed that one of the
heads, instead of shriveling up, had fallen off onto the sand bed. I
gently picked it up (with gloves on of course!) and relocated it on top
of one of the live rocks. It doesn't appear dead. <AHA!
We're on to something, Watson! This is a rare event- total polyp
bail-out. LPS corals will do this as a last-ditch survival strategy
when they simply cannot survive any longer in a given environment. Be
it from lack of light, poor water, bad neighbors, this is an attempt to
ride the current to somewhere that they can establish a new
skeleton.> Today, I decided to rotate the colony a bit so that the
heads pointed more upward. In doing so, though I was trying to be
careful, I accidentally knocked one of the heads against the tank wall
and then dropped the colony "head down" onto the sand bed! I
quickly picked it up and shook the loose sand off the polyps. I
sincerely hope I didn't just kill it!!! Edit: As I type this, the
head that had hit the sand is starting to extend its polyps once again,
so I'm taking it as a good sign. <These corals are tougher than
we give them credit for. I'm sure they'll be fine with your
continued attentive care.> Finally I should mention that the colony
is a good 5-6 inches from the colony of Zoanthus, and I've never
seen the two physically touching. <And there lies the rub: They
don't have to. Search WetWebMedia re allelopathy and you'll
learn all about the noxious chemicals cnidarians leach into the water
to guarantee the demise of their competitors. Can I venture to suspect
that the first polyps to die may be those in water current that has
recently passed the Zoanthus colony?> So basically, my question to
the crew is, are there any other steps I can take to try to forestall
the continued decline of this coral? Should I break or cut off the old
dead branches? What should I do with the head that has fallen off?
<Make sure the head is somewhere it won't be macerated by the
current, and it may survive. It is my suspicion at this time that the
Zoanthus have become stressed or sense the presence of the torch coral
and are beginning to exude extra toxins, or the low levels they have
been emitting since November have built up to toxic concentrations. I
would run a little carbon in your filter and change some water to help
with the likelihood that this is the case. That said, let us know about
your water parameters and the proximity of the dead polyps to the
Zoanthus (or a current that just passed them) and anything else you
feel may pertain to the information you read on allelopathy.> Thanks
so much for your time. I've tried to describe the situation as
detailed as possible, since I don't have my camera here with me.
Let me know and I can try to attach pictures. <Thank you for taking
the time to write such a descriptive email- it really helps us answer
your question promptly and accurately.> Thanks again! <You are
very welcome!> Dan <Benjamin>
Re: Torch coral declining - 6/4/08
Benjamin, <Hello again!> Thank you so much for your quick reply!
I would like to answer several of your questions and also report on the
health of my torch coral, which seems to have improved quite a bit
since just yesterday. <Glad to hear it!> I did a water change
yesterday and added some buffer to the tank to continue to raise
alkalinity (Seachem Reef Buffer). This morning, when I came in to the
office, all but two of the remaining live heads of the torch coral were
extended, and more so than I've seen them in days. Also, the head
that "bailed out" is sitting on the rock and seemingly doing
ok for now. Needless to say I was very encouraged. To answer your
questions about water quality, here are the numbers which I tested this
evening: Ammonia: 0-perhaps a trace. I measured this just after
feeding, and didn't read the color until a little after 5 minutes
(this was using the API two-reagent test kit), but it may have been
ever so slightly green. I will keep an eye on this to make sure it
doesn't get beyond this. It has shown this ever-so-slight green
color before, which is so hard to tell from the reference zero color,
depending on the angle and brightness of the local light, that I
haven't worried too much about it. <Color-based tests are,
unfortunately, sometimes quite hard to read> Nitrite: 0 Nitrate: 0
Calcium: 420 ppm KH: 10 (getting better!) pH: 8.2 <Looks good. pH
could be higher in ideal conditions, but 8.2 shouldn't cause
problems as far as I know.> Otherwise, to answer your question about
which heads die first, it doesn't appear to be related to which
heads are in the path of the current that has just passed the Zoanthus
colony. In fact, two of the heads which appear to be the first to get
this current are two of the ones that are continuing to do well. Thus,
at least as far as I can tell, there doesn't appear (at least on
the surface) to be too much allelopathic competition between the two
colonies (yet). I unfortunately have ran out of filters, so I need to
buy a few from the LFS, which I should be able to do in the next couple
of days. These are pre-made for this particular aquarium and already
have carbon in them. I suppose I could also get a separate filter bag
and put some carbon in. <Okay. I still wouldn't rule out some
sort of allelopathic interactions, but it is good to know they
don't seem to be directly harm each other.> I should also
mention that along with the Zoanthus, I have some sort of purple
anemone which I'm pretty sure is a type of Aiptasia growing in the
middle of the colony (I don't know why I forgot to mention this
yesterday). It tends to keep the Zoanthus polyps nearest it closed,
presumably because it's stinging them with its tentacles, but
it's pretty enough that I'm loath to kill it. <That could be
problematic. Stressed Zoanthus will exude toxins into the water,
although they vary in potency based on the species, environs, etc. If
the torch continues to decline after alkalinity has been brought up and
the coral is being fed regularly the next step might be to deal with
de-stressing your Zoas.> If I could relocate it safely, I would, but
I hear that these guys tend to get out of control! Again, sorry I
don't have any pictures (yet). I have my camera today but forgot
the USB cable to transfer the pictures! <That's okay. I am
curious about this anemone nestled in your Zoanthus, but I think for
now we can chalk this up to starvation/water quality and dig more into
allelopathy if there continues to be decline.> Otherwise, given the
improvement in the torch colony since just yesterday, I feel that the
low alkalinity was an important factor. However, come to think of it,
since I also did a water change, I can see how removing some of the
toxins from the Zoanthus could also have been just as important. I will
certainly keep you posted over the next few days with the status of the
colony. <Sounds good. I hope all continue to improve!> Again,
thanks for your quick response, it is much appreciated! <No
problem!> Dan <Benjamin>
|
Torch Coral Problem -- 04/01/08 First off,
good evening to all. <<Greetings>> I have 4 different
torch corals in my tank. Recently, while cleaning I broke one of
the heads off the base and thought nothing of it because I did
hit the coral. Tonight I looked in my tank and noticed on another
torch coral one of the branches was deteriorated to where it was
almost separated from the rest of the polyps. All of the polyps
in my tank are extremely healthy and fed 3 to 4 times a week.
What would be causing the branches to be so brittle or what could
be eating the branches? All my levels are good, the system has
been running for about 1 1/2 years and I would say this is the
first time I've noticed this. All the torch corals were
purchased over a year ago and have doubled in size. Any help
would be appreciated. <<Hmm, you say 'all levels are good''¦what
does this mean, exactly? Have you tested bio-mineral content (Calcium,
Magnesium, Alkalinity)? How often do you perform water changes? How much
do you change? How large is this system? Do you have any other stony
corals? Are they malaffected as well? I can only guess that your system
is deficient in bio-mineral content and is 'robbing' this back from the
coral's skeleton. If this is not the case, then perhaps a boring
sponge'¦ Regards, EricR>
Re: Torch Coral Problem -- 04/01/08 Thank
you for the response. <<Quite welcome>> I do test
Calcium and Alkalinity but have never tested Magnesium.
<<Ah! The three do 'perform' together. If Magnesium
is deficient (should be approximately three times the Calcium
level) it can cause difficulty with maintaining sufficient
Calcium and Alkalinity levels which can certainly cause the
coral's problems with building their skeletal
structures>> I perform 80 gallon water changes on my +-300
gallon tank every three weeks. <<Sounds good>> All
the other stony corals are doing fine and I haven't noticed
any change. <<Hmm'¦perhaps the lighter/less dense
structure of the Torch Coral skeletons means they are 'the
first to go'>> I do notice these sponge looking growths
by my overflow...they're about 1 inch in diameter,
½ inch think and I have about 5 of them. I didn't
think they would do any harm so I haven't removed them.
Unfortunately, I cannot take any pictures of them where
they're located. <<I don't think it likely these
organisms are the/a problem>> Do you think I should dose
some trace elements? <<Not without testing for the need
first'¦but I would think your water changes provide
these just fine>> I am running a dual chamber calcium
reactor at 1 bubble per second so I wouldn't think I need to
add additional calcium. <<Maybe'¦maybe
not'¦ If the tank is heavily stocked, bio-mineral
content may be depleted faster than you realize. But this can be
evaluated with tests performed a day or two apart for a week or
so to determine how quickly the tank is utilizing the available
bio-minerals. At the least, do make sure the water
chemistry/bio-mineral content is 'in balance'>>
Once again, thanks for the response. <<Cheers,
EricR>>
|
|
Bubble Coral Disease? (Or Maybe Environmental
Issues) -- 02/02/08 Hi Eric! <<Morning Don!>>
Sorry to bother you again but any chance of telling me what's
going on with this Bubble coral? I have had it for about 2 months
and the last week it has been looking like this. Thanks again.
<<Hmm'¦It is hard for me to discern much from this
photo (too small, too distant), but it appears the coral is
experiencing polyp bailout. This is usually a result of
stinging/poisoning from another coral in too-close proximity, a
result of 'light-shock' (either from being placed too
high in the aquarium or as a result of new bulbs, or maybe just
clarifying of the water from the addition/changing of filter
carbon), or a result of a decline in water quality or an
imbalance/deficiency of Earth/alkaline elements. If the coral is
not too close to another (or not being harassed by a fish), and
if lighting is not the issue, then look to your water
quality/chemistry. Ensure Nitrates are below 5ppm and that
Magnesium/Calcium/Alkalinity are all within NSW levels.
Also'¦have you been feeding this coral? Plerogyra
species are quite voracious predators and usually require
supplemental feeding for their long-term wellbeing (as do most
ALL corals, in my opinion). Small meaty foods like frozen mysis
(twice a week) are a good supplemental food for this coral.
EricR>>
|
|

|
Torch Coral... hlth.
9/12/07 Hi Bob, <Hi Cameron, Mich filling in tonight.> I
have a torch coral that I have recently noticed missing two polyps
next to each other. <Aye! Doesn't look happy!> The last
time I noticed tissue there was around 3 - 4 days ago. Is it
possible that it could disintegrate that quickly without my
noticing? <I highly doubt that it disintegrated without you
noticing... it's usually a pretty nasty process. Trust me, been
there done that. Sometimes when the coral is really unhappy it will
bail its polyps. I suspect that's what may have happened here.
It is possible for the polyp to survive detached from the skeletal
base, but it is uncommon. But if you look in the nooks and crannies
of your tank you may find these two missing heads floating around
somewhere in the LR. More here:
http://www.wetwebmedia.com/carydisfaqs.htm
http://www.wetwebmedia.com/corldisfaqs.htm > Or perhaps some
other explanation? The rest of the coral appears healthy and
normal. <Mmm, I don't know that I would say that. Looks
rather deflated and unhappy to me. This coral should be fuller and
fatter. I suspect allelopathy is at work here. You may need to
relocate this coral or one of its neighbors. More here:
http://www.wetwebmedia.com/carycompfaqs.htm A water change and
adding carbon likely wouldn't hurt either. I would also check
your calcium levels. I suspect they may be low.> I hope you
receive my photo ok. <Yes.> Also I have a question regarding
Tubastrea (sun coral) feeding; I have been using Tropic Marin's
Pro-Coral Zooton. A substitute for zooplankton feeders, is this
enough to sustain the coral or continue with feeding with Mysis
shrimp? <I am not familiar with Zooton, which makes me question
it's nutritional value, I suspect you would have more success
with Mysis or other finely minced fresh seafoods or if you're
looking for something prepared then perhaps Cyclop-eeze. If you
really want to go crazy with feeding see the method employed here:
read about Re: Feeding of Tubastrea.. Follow up to Baby Tubastrea
Timeline 8/7/07 on this page:
http://www.wetwebmedia.com/dendroreprofaqs.htm > Cameron Teague
Tasmania, Australia
<Michelle Lemech
Pennsylvania, USA> |
|

|
Bubble coral problems 9/2/05 Hello, I've been
an avid reader of your site for almost as long as I've been in the
saltwater fish world (it took me a while to find your site!) and
it's been a world of help - and now I need your help again.
<Glad to hear!> I've had a good sized (about 4 inches in
diameter shriveled) piece of bubble coral. Over the past two
months or so, it has spent most of its time 'shriveled' up, and
the tissue has started receding and more and more of the skeleton is
becoming bare. When I got it the 'stem' was almost
half covered with tissue, now, there's no more tissue on the stalk
at all - just the top. Sometime the coral will be fully
expanded for a whole day, and then boom, shrinkage city for another
four days. I have a candy coral that's doing great, as
well as a red bubble tip anemone int he tank that's
thriving. Nitrate, nitrite and ammonia are at
0. My ph is 8.0 and my Ca is 420... Any ideas on
why my poor coral is slowly dieing? Please help! -Pauli
<A few things come to mind. First... if you have a lot of
mushroom anemones or large soft corals, they may be producing a lot of
defensive chemicals. Second, you did not mention
alkalinity. Alkalinity is just as important as calcium, but
often neglected. Lastly, nipping fish. Dwarf angels,
blennies and even some tangs can be culprits here. Hope this
helps. Best Regards. AdamC.>
Torch coral shedding tentacles 6/13/03 Feeding my torch coral
recently with a baster, I noticed some tentacle tips drifting free. I
gave it a good blasting to free all the dead tips, there were a lot of
them. The 'dead' tissue is the ball-shaped tentacle tip with
about 1/4 inch of tentacle tissue. It appears to replace the shed tips,
since its overall appearance hasn't changed, it still looks good. I
can't detect any tentacles with missing tips. Q: Is this normal?
<hard to say from your description... tentacles can be shed as a
natural reproductive or defensive strategy, or (more often) as a sign
of poor health/infection> This specimen is sitting about 6 inches
below PC lamps (8000K and actinic), these lamps are about 10 months
old. I feed it Tropical Crisps or other flake foods ground into powder,
and sometimes Kent Microvert. <do send a picture if possible. Close
up to see if there is any necrosis in evidence. Else we can only
speculate from the general description I fear. Best regards,
Anthony>
Euphyllia parancora question 6/11/03 hello there,
<howdy!> I have a 120g tank with mixed soft corals and a few hard
corals. Everything is fine except I have spotted that my beautiful and
large Euphyllia parancora which is expanding very well and swelling
enormously seems to have a part of the skeleton exposed. <the
swelling large could be a bad sign if water clarity or light intensity
have degraded over time. Causes corals to pan for the waning light yet
give the appearance of "good health"> Now all around the
colony the flesh of the coral does not simply come out of the ridges
but extends further down each coral head also I can see a demarcation
where the flesh starts even when the coral is 'resting' -
although it never retracts its tentacles. One small section of this
ribbon of flesh that extends for about one inch around all the coral
heads appears missing and I can see the whiter skeleton. Extension is
very good all over the colony but this thing bugs me. Could it be the
start of something more sinister? In that case what precautions should
I take? Tank you very much for your ever speedy responses. Massimo
<its difficult for us to say with little information on your
tank/history/husbandry and no picture provided. Do consider the
overextension issue raised above if your lights are over 10 months old,
if the lamps or lenses are not cleaned of dust and salt creep weekly,
and/or if water clarity (lack of weekly/monthly water changes and
carbon). Do send a pic if you can. Best regards, Anthony>
Spots to left of me, bubbles to the right - 2/6/03
Hi to all, <Huuulllllooooooo.> I'm
wondering if the brown, translucent spots on my white bubble coral are
of any concern. <Sounds like a Planaria infestation, but could maybe
be the start of some sort of "Brown Jelly" issues maybe?
Other than the spots, are you noticing any disintegrating tissue?>
The tank is 6 months old, water parameters are great, temp 80, sal. 20,
<Do you mean 1.020?> lighting is a 48"PC <what kind of
light? Just curious> which are on 8-9 hrs daily, water
changes are 3-5% wkly <Mmmm.....maybe 5-10% weekly would be
better> and the tank is 55glns. The coral is a little more than half
way down, the brown spots started about two to three weeks ago and is
covering approximately 75%. <A picture would be really helpful here,
but if it seems that these are small irregular looking spots some
darker than others, then try gently blowing bubble coral with a turkey
baster. See if these "spots" come off or move.> Its fed
twice a week with Mysis shrimp and a home blend food which includes
garlic, serving size is less than 1/4" or smaller. <Could be
fed more. Is it still eating currently?> There is a torch coral,
<Be sure that the Torch is far from the Bubble as they have a
tendency to use their feeding or err....."sweeper" tentacles
to wage war on other corals, animals, and yourself <G> when not
feeding with them> purple mushroom, buttercup and a plate
coral <Be sure this coral is not too close to anyone either. As a
matter of fact be sure they are all pretty far apart if not already
;)> in the tank with it. <Do any other corals have any
"spots"?> I do have two gold band maroon clowns in the
tank which don't bother it at all, actually I don't see any of
the fish bothering it. Any thoughts? < Hard to say. See above
suggestions. I would check here also: http://www.wetwebmedia.com/pestflatwrmanthony.htm
http://www.wetwebmedia.com/flatworms.htm
http://www.wetwebmedia.com/corldisfaqs.htm
Hopefully something in there will help identify the issue. Let me know
if I can be of more help. If you have the means, please send a pic.
Paul >
Bleached hammer coral Hi I have a 29 gallon mini reef that
has a branched hammer coral that turned white a few months ago.
<likely from a attrition (starvation from lack of light and/or lack
of target feeding) or salinity/temperature shock. The latter can occur
and not effect all coral... different tolerances with each. Do you
recall a sudden increase in heat or a lapse in evap top off followed by
the dumping of a sudden large amount of freshwater in to compensate? If
not... the coral was simply starving... very common. Many poorly lit or
underfed coral can go 6-12 months before finally waning noticeably>
It doesn't open as large as before but otherwise it seems fine. It
has been like this at least 6 months. <Yikes> The mushrooms
and other corals seem fine. Water quality is fine. I have one power
compact SmartLight and one 20 watt triton regular fluorescent about 4
inches above the glass top. <that's your first problem, my
friend. All fluorescents need to be 3" or closer to the water to
be remotely useful. Your lights are already modest and the glass canopy
filters even more light... especially if it has had salt creep or dust
on it prolonged. Not enough useable light has been getting to this
animals to help it feed itself photosynthetically. See this article
here: http://www.wetwebmedia.com/marlgtganthony.htm Furthermore these
Euphyllia species need more food than most coral to support symbiosis.
Feeding 3-5 times weekly with fine meaty food is necessary... see this
article here: http://www.wetwebmedia.com/fdreefinverts.htm> Any idea
what might be wrong or what I can do to get some color back in it?
Thanks . <the main thing is to use fresh bulbs (6-10 months old,
keep clear water (carbon changed monthly is not small amounts weekly)
and feed daily in small amounts until color returns and then you can
back off just a little. Best regards, Anthony>
Pearl bubble coral question Hi, <Anthony Calfo in your
service> My pearl bubble coral (had it for 2 years and
growing/eating ok) has developed today something quite unusual. At
first I thought it was a bit of algae or something stuck in it but when
I looked better it looked like 2 stalks of tissue protruding at each
end of it dark in color and quite long and straight/still, not like the
ones with nematocysts seen mostly at night) and culminating in a small
transparent pocket. Never seen anything like it before. I thought it
might be reproducing... Any ideas as to what it might be? <several
things... a photo might help too if you can. LPS corals commonly
produce polyp balls as a reproductive strategy. A modified tentacle on
a polyp becomes incused with a calcareous nodule. This daughter
satellite continues to grow until the calcareous "stone"
inside becomes sufficiently mature and weighted to tear away from the
parent and begin life as a free living division, soon to attach to the
reef (hopefully). The event could just as easily be stress though most
often from a change in lighting (sudden change of carbon/chemical media
after a long period without which suddenly improves water clarity and
light penetration, cleaning salt creep on lenses or bulbs, and of
course new lamps). Excessive illumination may cause photoinhibition or
the excess production of O2 in the tentacle by over stimulated
Zooxanthellae. Indeed... there are several possibilities. Time will
tell... do consider the above and possible light shock just the
same> Thanks, Massimo, Brighton UK <kindly, Anthony Calfo>
Re: Pearl bubble coral question Thanks for the amazingly
prompt answer. <We aim to please, my friend> I have just
observed the coral slowly retracting first one then the other of the
protrusions shortly after lights out. Probably stress, as you mentioned
-today I changed 10% of the water as part of my weekly routine-. The
coral appears otherwise to be fine and in the usual state. Thanks
again. <very well. Do focus on maintaining stable water quality. Be
on alert for any color changes (particularly paler color). Be sure to
feed as well or better than before: this animal should be fed finely
shredded meats of marine origin no less than three times weekly... it
is heavily dependant on feeding. No worries, though... Bubbles are
generally quite durable and adaptable. One other consideration would be
a change in current. They are disturbed easily by stronger water
movement that is otherwise good for most coral. If you have increased
flow recently (cleaned or added power heads/pumps) this perhaps has
contributed.> Massimo, Brighton UK
<best regards, Anthony>
What's That On Your Hammer? Eeewww!!! Hi, I have a
tri-color hammer branch that had been doing quite well for a couple
months (that's about how long I've had it) but then I added in
a frogspawn coral on the other side of the tank and started adding in
calcium and iodide in moderate quantities. Since then, the hammer has
been almost completely closed up. The frogspawn, meanwhile, is
flourishing. Over the last week or so, I've noticed that long
stringy brown algae has been growing on the hammer and I started moving
it away but probably not very effectively because it always came back.
Someone at my LFS recommended using a turkey baster which appeared to
literally blast away all the bad algae and maybe some brown stuff that
seemed to be inside the hammer. That very night (yesterday), the hammer
started coming out again, probably to about 50% of what I've ever
seen it at but then stopped and I noticed some small pieces of algae
growing on the edges. I blasted those away too (though rather gently so
as not to harm the hammer) though the hammer didn't come out any
more. However, this morning, more brown stringy algae was on the hammer
and the hammer had pulled back into itself. Is my hammer damaged
or diseased? Is there a way to get rid of the algae from growing on it?
I'm relatively certain that if I could get the algae to go away,
the hammer might come back out as normal. I have 3 blue-leg hermits,
one Astrea snail and one turbo snail, but recently (last couple weeks)
I have noticed that the brown algae on the glass seems to be a little
out of control as well as some red slime algae on the substrate.
Thanks for all your help! Veronica <Hi Veronica, The algae (which
I'm guessing is Cyano bacteria from your description) is growing on
a dead surface, meaning that the hammer is most likely dead in the
areas which the algae is growing on. Your regular additions of iodine
may have caused this, as well as moving it. I would recommend you
purchase an iodine test kit and test for your iodine levels. You should
always test for anything you're adding. Blasting the Cyanobacteria
off the hammer is a good idea. Cyanobacteria (or, also referred to as
Red Slime) is usually caused by lack of currents and extra nutrients.
Overfeeding could possibly lead to the Cyano taking over corals.
Phosphate will also elevate your Cyano levels. For now, I would
continue to blast the algae off the hammer and discontinue dosing
iodine until you've tested for it. I would also look into feeding
less and adding more current to your aquarium to prevent further Cyano
build up. Take Care, Graham>
|
|

